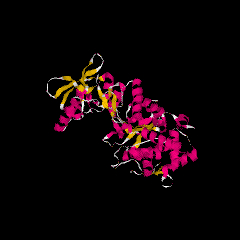
François JACOB
Friends or foes: Adenylyl cyclases and guanylyl cyclases
Antoine Danchin ©
Cyclic AMP is ubiquitous in the regulation of enzymatic activities or gene expression in all domains of life, except for Archaea. This explains the interest in its mode of synthesis and the extensive literature on the enzymes that produce cAMP, adenylyl cyclases (ACs), often known as adenylate cyclases, adenyl cyclases or ATP pyrophosphate lyases (EC 4.6.1.1).
These enzymes carry out the synthesis of cAMP from ATP and produce pyrophosphate as a by-product. They can be grouped into five classes based on their sequence characteristics. These include:
The first complete adenylyl cyclase (formerly
adenylate cyclase) gene was cloned
and sequenced
from the model bacterium Escherichia coli. ![]() It is located near the origin of replication of the organism, in an
area of the genome involved in iron metabolism. Molecular genetic and
biochemical studies have shown that E. coli adenylyl
cyclase consists of two domains. The catalytic domain is NH2-terminal
whereas the glucose-sensitive regulatory domain is COOH-terminal.
Its function is still enigmatic at the end of year 2025. Studies on other enterobacteria, such as Erwinia
chrysanthemi, Proteus mirabilis, Salmonella
typhimurium, Yersinia intermedia and Yersinia
pestis have shown that both
the gene context and the proteins encoded at the cyaA locus are similar in size and overall organisation to those of E. coli. The downstream gene
expressed in the opposite orientation, cyaY, is involved in
iron metabolism, it has an analog in Eukarya, frataxin, the defect of which
is responsible for Friedreich ataxia in humans. Analysis of the cyaA
gene, using either complementation of appropriate cyaA-defective strains of E. coli or direct sequencing of whole genomes, showed that other bacterial species, including Aeromonas caviae, Aeromonas
hydrophila, Haemophilus influenzae, Pasteurella
multocida, or Vibrio cholerae, related to Enterobacteria but
distinct from them synthesise a protein that is structurally
and phylogenetically related to the E. coli cyclase.
This gene is absent from many Bacteria, including
gamma-proteobacteria such as Xanthomonas campestris, or Pseudoalteromonas haloplanktis. However it is present in Pseudomonadales.
It is located near the origin of replication of the organism, in an
area of the genome involved in iron metabolism. Molecular genetic and
biochemical studies have shown that E. coli adenylyl
cyclase consists of two domains. The catalytic domain is NH2-terminal
whereas the glucose-sensitive regulatory domain is COOH-terminal.
Its function is still enigmatic at the end of year 2025. Studies on other enterobacteria, such as Erwinia
chrysanthemi, Proteus mirabilis, Salmonella
typhimurium, Yersinia intermedia and Yersinia
pestis have shown that both
the gene context and the proteins encoded at the cyaA locus are similar in size and overall organisation to those of E. coli. The downstream gene
expressed in the opposite orientation, cyaY, is involved in
iron metabolism, it has an analog in Eukarya, frataxin, the defect of which
is responsible for Friedreich ataxia in humans. Analysis of the cyaA
gene, using either complementation of appropriate cyaA-defective strains of E. coli or direct sequencing of whole genomes, showed that other bacterial species, including Aeromonas caviae, Aeromonas
hydrophila, Haemophilus influenzae, Pasteurella
multocida, or Vibrio cholerae, related to Enterobacteria but
distinct from them synthesise a protein that is structurally
and phylogenetically related to the E. coli cyclase.
This gene is absent from many Bacteria, including
gamma-proteobacteria such as Xanthomonas campestris, or Pseudoalteromonas haloplanktis. However it is present in Pseudomonadales.
The protein sequence showed no long stretch of hydrophobic amino acid residues, which suggested an explanation for the proteins' known membrane localisation. In all cases, the proteins were found to be very rich in cysteine residues, which is an unusual feature for proteins located in the cytoplasm or at the cytoplasmic membrane boundary. This may account for the extreme difficulty in purifying the enzyme. Additionally, the proteins are rich in histidine residues. This could indicate the involvement of metal ions in the folding and/or activity of the polypeptide chain, though there is no experimental data to support this hypothesis. Finally, the protein consists of two functionally well-defined domains. Comparing the polypeptide sequence of the catalytic domain of the E. coli enzyme with known sequences in protein data libraries revealed no significant similarities with other known proteins. The catalytic domain sequence is approximately 420 residues long and has been identified experimentally. Differences in amino acid residues in the Erwinia chrysanthemi enzyme compared to its counterparts are often due to the presence of complementary charged residues instead of neutral ones. This suggests that ionic interactions (including salt bridges) are more prevalent and stabilise the protein at this bacterium's lower growth temperature. This observation could be useful in understanding the tertiary structure of the protein, in particular after its sequence has been generated using Alphafold.
The carboxy-terminal domain of the protein is involved in regulation of the enzyme activity, in particular in its inhibition in the presence of glucose. A component of the phosphorylation cascade mediating import of glucose in the cell, enzyme IIAGlc has been proven to be involved in regulation, in a way not yet understood. An aspartate residue (D414 in the E. coli enzyme), appears to be involved in the process, in an unknown way. A reflection on the structure of the enzyme suggests that tonic inhibition of the catalytic domain by the regulatory domain could be relieved by phosphorylation (or methylation) of this residue (modification has never been demonstrated, however).
Whooping cough is caused by the Gram-negative bacterium Bordetella pertussis, which secretes into the medium many toxic proteins, including an adenylate cyclase. In 1980, it was discovered that this AC is activated by a host protein, calmodulin, that does not known to occur in bacteria. Two years later Leppla demonstrated that another toxic AC, secreted by a Gram- positive bacterium, Bacillus anthracis, the causative agent of the infamous anthrax, was also activated by host calmodulin. These observations stimulated intense efforts, but despite numerous attempts, it took several years before the cyaA genes from either organism could be cloned. However in 1988, the cloning and sequencing of AC genes coding for the calmodulin-dependent cyclases was made possible by a simple idea, predating its generalisation under the name of the "double hybrid technique": in vivo complementation by a plasmid encoding an activator of the function [in this case, calmodulin (1988a, 1988b)]. Cloning of a toxin from such a dangerous organism raised ethical concerns that were not taken seriously into account until many years later.
Bordetella pertussis AC is synthesized as a large bifunctional polypeptide of 1706 amino-acid residues. Different authors had however published various low values for the purified protein molecular mass (from 43 to 70 kDa). This fluctuation in biochemical data was understood when it was demonstrated that the N-terminal segment of the protein (400 residues) displays calmodulin-activated AC activity, while the rest of the molecule was responsible for the hemolytic activity and the secretion of the toxin in the external medium. Sequencing, molecular genetic and physiological studies indicated that the AC domain was fused to a polypeptide similar to the polypeptide chain of E. coli hemolysin toxin. The name cyclolysin was therefore coined for the toxic adenylate cyclase from B. pertussis. The AC of B. anthracis has been named after the symptom it triggers in the infected host, edema factor (EF). It is encoded in a plasmid, together with another toxin, the letal factor (LF) and a carrier protein, the protective antigen (PA), which is necessary for internalisation of both EF and LF into host target cells. The AC (EF) protein, 800 amino-acid residues long, comprises four regions of different function. The first region is a signal peptide, permitting secretion of the protein. The second region corresponds to the domain permitting binding with PA. The third region encodes the adenylate cyclase function. It is followed by a region of unknown function. These toxic ACs have been subjected to a most thorough biochemical analysis. The enzyme from B. anthracis has been crystallized and its 3D structure determined.
In spite of several attempts to isolate other members of this class we know only three examples of such proteins, isolated from extremely distant bacteria, a Gram positive one and two Gram negative (the AC from B. bronchiseptica is very similar to the B. pertussis enzyme). Comparison between the catalytic region of the B. pertussis and B. anthracis ACs identified four conserved regions that are involved in catalysis, calmodulin binding and activation. The first region comprises a Walker-type peptide, G----G(A)KS, similar to the nucleotide binding region found in many ATP or GTP binding proteins. It was therefore proposed to be part of the catalytic site. In vitro mutagenesis substantiated this interpretation. A second region, PLTADID, displaying some similarity with 6-phosphofructokinase, was also shown to be involved in catalysis, and it was proposed that the aspartate residues present in this region are involved in ribose and magnesium-phosphate binding. However, in spite of strong conservation, the first proline residue does not seem very important since it could be replaced by a leucine residue without any measurable influence on the activity or calmodulin activation of the wild type enzyme.
Although many calmodulin-dependent enzymes have been identified, the mechanism of activation by calmodulin is still poorly understood. In several cases, limited proteolysis released active calmodulin-independent forms of enzymes. Accordingly, it was proposed that the calmodulin-binding domain of these enzymes blocks the access of substrates to the active site and that activation results because following binding of calmodulin an inhibitory domain is removed. The most original feature of the B. pertussis protein is that it can be split into two separate domains that can recover most of the initial activity when put together. This observation together with analysis of mutants in the region conserved between the B. anthracis and B. pertussis enzymes, indicates that the proteins may form a catalytic center from the cooperation of two halves, the function of calmodulin being to trigger the appropriate conformational move necessary for the formation of an active catalytic center.
Remarkably, class II adenylate cyclases also exist in Yersinia species, as well as in Pseudomonas aeruginosa. It has now been shown that the activation protein is F-actin, while it is profilin-G-actin in Vibrio vulnificus.
Adenylate cyclases from multicellular eukaryotes have long been difficult to study because purifying the corresponding catalytic subunit is extremely challenging. However, following intense work all over the world, they have been the first ACs to be crystallised and analysed using X-ray diffraction studies. The activity of these enzymes is subject to a complex regulatory pattern, particularly by GTP-binding proteins. This will not be discussed here. Class III enzymes were first discovered in yeast, but for the sake of convenience, we will start with bacterial enzymes. Among the more recent discoveries is the regulation of some soluble enzymes by bicarbonate.
Class III ACs forms a very diverse collection in eubacteria, both in length and in regulation. Gram positive bacteria such as Corynebacterium liquefaciens secrete a large quantity of cAMP. This is due to a very active class III AC, requiring the presence of pyruvate for activation. Streptomyces coelicolor synthesizes a much less active AC that is involved in aeromycelium formation. Gram-negative bacteria such as Rhizobium meliloti synthesize several different ACs (disruption of both genes at the same time did not alter cAMP production, suggesting the presence of more ACs). The Gram negative sliding bacterium (myxobacteria), exhibiting an elaborated differentiation pattern, Stigmatella aurantiaca, harboured at least two AC genes, each of them corresponding to class III enzymes. Another myxobacterium, Myxococcus xanthus also has several such enzymes. These latter enzymes have been partially purified and shown to be inhibited by adenosine, as are the mammalian enzymes (see below). They comprise two domains, the catalytic domain being carboxy-terminal and the regulatory domain, a likely ion transporter in one case, and the phosphorylated moiety of a two-component regulatory system in the other case. Many other Bacteria possess class III cyclases, in particular cyanobacteria. These enzyme generally comprise two domains, the catalytic domain being carboxy-terminal. There is no indication that they must oligomerize to be active. A remarkable situation is presented with R. meliloti and its many cyclases, while Mycobacterium tuberculosis has probably as many as 15 such enzymes. The product of gene Rv1625c in this organism displays adenylate cyclase activity. The enzyme has been crystallized and preliminary data allowed the authors to propose a 3D structure.
Saccharomyces cerevisiae was the first organism in which class III AC genes have been cloned and sequenced. The enzyme is activated by the RAS gene product. Two forms of the enzyme may exist, a long form containing repetitions of a leucine rich motif that plays a regulatory role, whose significance was recently substantiated and extended. It was clear then that this eucaryotic AC was completely different from the enterobacterial class, not only because of the sequence difference in the catalytic center, but also because the organisation of the gene was different: the catalytic domain is located at the COOH-terminus in S. cerevisiae cyclase, whereas it can be found at the NH2-terminus in E. coli. The yeast enzyme remained the only instance of its class until Garbers, Goeddel and coworkers recognized that the guanylyl cyclase coding genes that had been cloned from several metazoans, were derived from an ancestor in common with yeast AC. Another member of class III was subsequently discovered by Young et al. who cloned the AC gene from Schizosaccharomyces pombe by hybridization using the catalytic domain gene sequence from S. cerevisiae as a probe. Finally, the first higher eukaryote AC gene isolated in Gilman's laboratory from bovine brain, displayed features clearly reminiscent of this class. Since then many other genes or cDNAs for ACs belonging to this class have been isolated and sequenced from lower eukaryotes Saccharomyces kluyveri, Trypanosoma brucei and T. equiperdum, Plasmodium falciparum, Neurospora crassa, Dictyostelium discoideum, where it is involved in differentiation.
Many class III ACs have been identified in higher
eukaryotes, in particular in vertebrates, but the most thorough
study is in mammals, where several types, differing by their
regulatory properties have been identified All are regulated in more
or less complex ways by G proteins. Mammalian ACs have been
informally grouped into nine types, according to their tissue
location and activity regulation. All but type 9 are activated by
the diterpene forskolin, some are by protein kinase C and/or other
regulators. Type 1 enzymes were described as calmodulin activated
enzymes from brain, type 2 proteins are found in brain, lung and
other tissues, type 3 are abundant in olfactory tissue, and the
smaller type 4 enzymes are present in testicular tissue. AC 1, 2 and
8 are positively regulated by calcium/calmodulin, whereas types 5
and 6 are directly inhibited by calcium. ![]() AC 2 and 4 are sensitive to multiple regualatory inputs proceeding
from diverse receptors. AC9 mRNA is found in rat brain. It is
particularly abundant in hippocampus, cerebellum, and neocortex. It
should be noted however that the classification into types is
somewhat arbitrary (note for instance that type 4 can also be
calmodulin-activated). They all have overall similar structure, with
two phylogenetically related cytoplasmic domains required for
catalysis, connected by an integral membrane domain and variable
integral membrane domains at the NH2 terminus of the protein. Among
their many functions, their role in synaptic plasticity and memory
is particularly interesting, and will certainly make ACs again
extremely fashionable.
AC 2 and 4 are sensitive to multiple regualatory inputs proceeding
from diverse receptors. AC9 mRNA is found in rat brain. It is
particularly abundant in hippocampus, cerebellum, and neocortex. It
should be noted however that the classification into types is
somewhat arbitrary (note for instance that type 4 can also be
calmodulin-activated). They all have overall similar structure, with
two phylogenetically related cytoplasmic domains required for
catalysis, connected by an integral membrane domain and variable
integral membrane domains at the NH2 terminus of the protein. Among
their many functions, their role in synaptic plasticity and memory
is particularly interesting, and will certainly make ACs again
extremely fashionable.
The widely spread origin of class III ACs is reflected by the wide variation in their general organisation and molecular mass. The smallest protein is the R. meliloti enzyme, which appears to contain only a catalytic domain. Yet data exist, suggesting that an upstream sequence may yield a much longer protein having a complex regulatory pattern. The next shortest protein, also a bacterial one, is the enzyme from C. liquifaciens. The yeasts yield long proteins, as do higher eukaryotes (except perhaps in the case of the testicular enzyme). In all cases, the catalytic domain is located at the COOH-terminus. The mammalian enzymes consist in twelve hydrophobic membrane-spannning regions forming two distinct domains, and two cytoplasmic regions which contains both variable and conserved regions, and, in particular, two well-conserved domains that are responsible for catalysis .
Comparison of the catalytic domains sequence of the class III proteins showed that four amino-acid stretches, (M/L/I/V)(M/L/I/V)F(A/T)(D/S)(L/I)–(N/D)(F/S), (I/V)KT–G(S/D)(A/S/T)(Y/F)M, (M/L/I/V)(R/K)(M/L/I/V)G(M/L/I/V) (H/N)–(V/A)(V/A)(A/S)G, and (W/Y/F)G(N/D/P)TVN–ASR(M/L/I/V) are strongly conserved. Crystallisation of the catalytic core of the protein and determination of its structure by X-rays diffraction showed that these regions are part of the organisation of the structure. The crystal structure gives the forskolin binding site, but unfortunately not the nucleotide binding site. Further work involving co-crystals with G-proteins unraveled the nature of the substrate binding site. As a step towards understanding evolution and function of class III cyclases, enzymes displaying significant guanylyl cyclase activity having evolved from an adenylate cyclase ancestor were isolated. A single amino-acid residue change (GDTVN to GDTIN), in the region of the fourth alpha-helix of the catalytic core, altered the nucleotide specificity of the enzyme. This corresponds to a pocket situated in a region of the protein that is invoved in accomodation of the heterocyclic base.
Cyclic GMP is synthesized from GTP, yielding pyrophosphate as a product, by the enzyme guanylyl cyclase (guanylate cyclase, guanyl cyclase EC 4.6.1.2). CyclicGMP acts as an second messenger, activating cGMP-dependent kinases and/or regulating cGMP-sensitive gated ion channels. The role of cGMP as an intracellular messenger in vascular smooth muscle relaxation and retinal photo-transduction is well established. Garbers, Goeddel and coworkers found that the catalytic centers of guanylyl cyclases were strongly related to eukaryotic class III adenylate cyclases. Thus GCs form a single class, in contrast to adenylate cyclases, but are of at least two very different types, linked to the function of the enzyme in the cell. Guanylyl cyclase is found both in the soluble and particulate fraction of eukaryotic cells. The soluble and plasma membrane-bound forms differ in structure, regulation, biochemical and physico-chemical properties and constitute two distinct families. The currently known plasma membrane-bound forms (sensor-family) have been first identified as receptors for small polypeptide hormones. This first type is a complex, membrane bound, enzyme made of a receptor for specific hormones coupled to a catalytic domain similar in sequence and structure to the catalytic domain of class III adenylate cyclases.
Guanylyl cyclases in family I acts as sensors, and are often receptors for hormones such as atrial natriuretic peptide, involved in the control of osmotic pressure and sodium excretion in mammals. The known guanylyl cyclase receptors have been recognized as forming several subfamilies. Guanylyl cyclases receptors from the sea-urchins recognize speract and resact, which are small peptides that stimulate sperm motility and metabolism. The receptors for natriuretic peptides (ANF) exist in two forms that both synthesize cGMP: GC-A (also named ANP-A), which seems specific to atrial natriuretic peptide (ANP), and GC-B (or ANP-B) which are stimulated by brain natriuretic peptide (BNP) than by ANP. There seem to be at least three ANP receptors: two with GC activity (ANP-A and ANP-B) and one (ANP-C) which is responsible for the clearance of ANP from the circulation without a role in signal transduction. Intestine cells contain the receptor for the Escherichia coli heat-stable enterotoxin (GC-C). The endogenous ligand for this intestinal receptor seems to be a small peptide called guanylin. Odorant information is encoded by a series of intracellular signal transduction events thought to be mediated primarily by the second messenger cAMP. But a subset of olfactory neurons expresses a cGMP-stimulated phosphodiesterase (PDE2) as well as a GC of the receptor type (GC-D), demonstrating that cGMP has an important regulatory function in olfactory signalling. Finally, retinal GCs (often named retGC) exist in at least two forms, GC-E and GC-F. They play a specific functional role in the rods and/or cones of photoreceptors triggering a protein phosphorylation cascade. It consists of an apparent extracellular domain linked by a single transmembrane segment to an intracellular domain. It is coupled to GC activating protein-2, which is a Ca2+-binding protein that activates RetGC-1 in a Ca2+-sensitive manner. It is not known whether retGC acts as receptor, but its structure is similar to that of the other plasma membrane-bound GCs.
The organisation of all these GCs is similar: they have a N-terminal extracellular domain which acts as the ligand binding region, then a transmembrane domain, followed by a large cytoplasmic C-terminal region that can be subdivided into two domains: a protein kinase-like domain that is important for controlling the protein phosphorylation cascade linked to the specific signal recognized by the cognate GC, and a GC catalytic domain.
In contrast, the second family of GCs is cytoplasmic and soluble (hence the shorthand for soluble GC, sGC). It controls a completely different set of regulations. These cytoplasmic GCs form always heterodimers. The two subunits, alpha and beta are proteins which, although different in length (from 70 to 82 KDa) and sequence are highly related. Two forms of beta subunits are currently known: beta-1 which seems to be expressed in lung and brain, and beta-2 which is more abundant in kidney and liver. The most fascinating feature of these subunits is that they bind a heme prosthetic group. Upon binding of nitric oxide the sGC catalytic activity is stimulated, generating the intracellular signaling molecule cGMP. Discovered in 1987, NO is a signal transduction molecule, and its importance has been stressed by its role in blood circulation and cardiac muscle functioning. Carbon monoxide (CO) (which has been found in bacteria but does not have a clear cut function as yet) also seems to play a role similar to that of NO. Fifteen conserved cysteine residues on sGC have been mutated to serine by in vitro site-directed mutagenesis. All of the resulting recombinant enzymes were able to synthesize cGMP, demonstrating that they are not directly involved in catalysis, but mutation of two cysteines located in the N-terminal, putative heme-binding region of the beta subunit yielded proteins that were insensitive to NO, and lost their heme prosthetic group. In contrast mutation of the corresponding cysteines on the alpha subunit did not alter NO responsiveness, indicating that heme-binding is probably a specific feature of the N-terminal domain of the beta subunit.
In general, gene organisation revealed conservation in both types of enzymes, of the localization of at least one catalytic domain in the carboxy-terminal part of the protein, coupled to a variety (in length and in sequence) of amino-terminal parts. The membrane and cytoplasmic forms of GCs share a conserved domain which is fundamental for the catalytic activity of the enzyme. A similar domain is also found twice in the different forms of membrane-bound class-III adenylate cyclases from mammals, slime mold or Drosophila. A polypeptide consensus pattern detects both domains of class-III ACs as well as GCs: G-V-[LIVM]-x(0,1)-G-x(5)-[FY]-x-[LIVM]-[FYW]-[GS]-[DNTHKW]-[DNT]-[IV]-[DNTA]-x(5)-[DE].
The common origin between adenylate cyclases and GCs, witnessed by the apparent facility with which it was possible to build up a purine nucleoside triphosphate cyclase of broad specificity, might be relevant to phylogeny of the catalytic center of class III enzymes. This similarity suggested the existence of an ancestral purine nucleotide triphosphate cyclase as a precursor. As a consequence of this interpretation of sequence data, one may wonder whether evolution has not permitted the existence of some interlock between both nucleotides syntheses, a given cyclase being triggered, under appropriate regulatory conditions, to synthesise cAMP or cGMP alternatively. This could have been used in cyclic nucleotide-mediated controls existing in eukaryotes, and explain to the older observation that, in some cases at least, cAMP and cGMP concentration was varying in opposite way.
Three classes of non structurally related ACs poses already a challenging problem. It came out as a surprise, therefore, that Aeromonas hydrophila species synthesized another enzyme (a very small cyclase of 193 residues), that has an optimal temperature for activity of 65°C, and that was at least ten times more active than the class I AC present in the same organism. No function has yet been discovered for this protein. It has been found in various isolates of A. hydrophila. There has been one report of the presence of cAMP in Archaea, but this was later proven to be due to an artifact of the growth culture. It was therefore interesting to see that the sequence of AC from A. hydrophila was significantly similar to a gene product of Methanococcus jannaschii. The gene was expressed in E. coli, where it is toxic, but did not restore cAMP synthesis. Therefore nothing is yet known about the nature of ACs, if they exist, in Archaea, but we may expect that, at some point they might be discovered, and that they would belong to this new class. Interestingly, genome studies revealed that Yersinia species possess a counterpart of the A. hydrophila enzyme. Even more interestingly, sequence comparisons reveal that this class of proteins is related to metabolism of Thiamine-triphosphate, an elusive phosphoryl donor that may have predated separation between Bacteria, Eukarya and Archaea, and may have an important role in enzyme regulation, that is still almost entirely enigmatic. The 3D structure of the protein from Y. pestis revealed a novel fold, that spans the three domains of life.
Cotta and coworkers found that ruminal bacteria Prevotella ruminicola D31d produced detectable concentrations of cAMP. The gene for adenylate cyclase was cloned and expressed in a cyaA mutant of E. coli. The cloned P. ruminicola D31d gene was able to complement the cyaA mutation and permitted fermentation of lactose on MacConkey Lactose agar plates. Production of cyclic AMP in the E. coli clone was confirmed by radioimmunoassay. The 67-kDa protein was novel in that no amino-acid similarity was observed with other adenylate cyclases from Eukarya, Archaea or Bacteria. This is the first example of an adenylate cyclase gene identified from an anaerobic bacterium, stressing again the evolution by convergence of synthesis of the important mediator, cAMP.
The study of cellulose synthesis by Bacteria revealed that a novel second messenger molecule, bis-(3'5')-cyclic di-GMP (c-diGMP) discovered by Benziman in 1987, is extensively used by bacteria to control multicellular behavior, antagonistically controlling motility and cell adhesion. c-diGMP results from the condensation of two GTP molecules into the cyclic compound, in a reaction that is not completely understood. Diguanylyl (diguanylate) cyclases are widely spread enzymes that combine usually a catalytic domain, related to the structure of class III adenylyl and guanylyl cyclases, and a variety of modules sensing the environment, or acting as regulators. The catalytic domain contains a highly conserved pentapetide GG[DE]EF. Cyclisation releases two molecules of pyrophosphate. The crystal structure of an example of this enzyme has shown that the fold is similar to that of class III cyclases, but that the nucleotide binding is somewhat differing from the situation in its adenylyl or guanylyl cyclases counterparts.
Subsequently it was found in the mid-2000's that still another second messenger was present in many Bacteria and in Archaea (3'5')-cyclic di-AMP (c-diAMP). These mediators are involved in pathogenicity, via modulation of the bacterial response to their environment. In bacteria, cyclic-di-AMP regulates a great many processes including cell wall homeostasis, potassium uptake, DNA repair, fatty acid synthesis, biofilm formation and central metabolism.
Finally, mixed cyclic dinucleotides cGAMP (2′3′ and 3′3′) were found to have a role in a variety of processes. 3′3′-cGAMP was discovered in 2012 by Mekalanos and colleagues while exploring the role of a Vibrio pathogenesis island and much of the synthesis and degradation of the molecule has been deciphered since, together with its identification in several other bacterial species. Even more recently, in 2013, a cyclic dinucleotide second messenger, 2′3′-cGAMP was discovered in mammalian cells by Ablasser, Hornung and colleagues. The biosynthetic enzyme was identified. Its degradation enzyme, glycoprotein ENPP1, was isolated and its structure established, showing a role for a calcium ion.
The concentration of these metabolites is regulated by a variety of phosphodiesterases, and the final dinucleotide is degraded by nanoRNAses of various descents.
Nucleotides play a universal role in life, as components of nucleic acids, as forms in which chemical free energy is stored, and as regulators of gene expression or enzyme activity. Cyclic adenosine 3',5'-monophosphate (cAMP) plays a universal role in the control of gene expression as well as in the integration of metabolic functions. It is present both in Eukarya and in Bacteria. cAMP seems to be absent only from Archaea (but see the "adenylate cyclase" entry). Its presence was controversial in plants, but a work investigating opine catabolism in plants resulted in the cloning of a gene that appeared to specify cAMP synthesis in plants. It was however later reported that this work was a fake, but recent data suggest that plants make cAMP, probably via a chloroplast adenylate cyclase. Cyclic AMP has been reported to exist in cyanobacteria and in algae. This ubiquity explains the major interest displayed in its mode of synthesis, and the vast amount of literature devoted to the enzymes that produce cAMP from ATP, the adenylate cyclases we have just described. Because cAMP is a regulatory molecule it must be either excreted in the environment or inactivated in order not to accumulate. This is performed by 3',5'-cyclic-nucleotide phosphodiesterases (EC 3.1.4.17). These enzymes are generally specific for cyclic nucleotides (namely cAMP and cGMP), and sometimes specific for cAMP or cGMP alone. A variety of natural inhibitors modulate their activity (nucleoside triphosphates, pyrophosphate, and especially methylated xanthines, such as theophylline), and by a variety of processes involving protein phosphorylation and/or calcium. adenylate cyclases form four independent classes of enzymes and this raises the question of the origin of cyclic nucleotides as regulatory molecules, as well as their universal implication in regulatory networks. Because it is very polar, and negatively charged, cAMP does not permeate easily into cells (unless through specific transporters, generally poorly known at present). More lipophilic analogs such as N6,O2'-dibutyryladenosine-3',5'monophosphate are therefore used to modulate its concentration and mimic its effect in cell cultures ex vivo, but in vivo inhibitors of phosphodiesterase, or specific mediators (neuromediators in particular) are used for therapeutic purposes where cAMP concentration must be altered.
Cyclic AMP was discovered in 1958 by E. Sutherland, who obtained a Nobel prize in 1971 for this and other discoveries on hormone action. As he has himself written, it is within the scope of molecular biology that cAMP was discovered : "When I first entered the study of hormone action, some 25 years ago, there was a widespread feeling among biologists that hormone action could not be studied meaningfully in the absence of organized cell structure. However, as I reflected upon the history of biochemistry, it seemed to me there was a real possibility that hormones might act at the molecular level". Sutherland built up a cell-free system where well-known hormones could control glycolysis in vitro. Using this system he isolated a small thermostable molecule that was able to activate glycogen phosphorylase. Chemical analysis of the molecule permitted its identification as adenosine 3'-5' cyclic monophosphate. Synthesis of cAMP was shown to be the result of the action of an enzyme, adenylate cyclase, that generated cAMP and PPi from ATP, when activated by adrenaline. Since this pioneering work, the study of cAMP mediated effects required the identification of the structure, function and regulation of adenylate cyclases, the cAMP synthesizing enzymes. And, contrary to expectation, this did not yield a unifying picture of the role of cAMP, but, rather, demonstrated that this molecule has been used over and over again by living organisms for very different functions.
At the time of cAMP discovery, the aphorism of Jacques Monod and François Jacob, "what is true for Escherichia coli is true for the elephant", induced biochemists to try bacterial systems to unravel cAMP function. After the discovery of cAMP by Sutherland in 1958, Mackman and Sutherland demonstrated that glucose-starved Escherichia coli cells accumulated cAMP. Ullmann and Monod later established that part of the catabolite repression phenomenon was controlled by cAMP. This discovery raised hopes that the study of this mediator in Bacteria would help to understand what happens in Eukarya (even perhaps in higher eukaryotes). However it became soon clear that cAMP in Eukarya was generally, as found by Sutherland, a "second messenger" that was used as an intracellular relay molecule to the action of extracellular hormones, while it acted directly on transcription via its receptor, the Catabolite Activator Protein in E. coli. Study of the slime mold Dictyostelium discoïdeum revealed another function of cAMP, phylogenetically linked to is hormone-mediated action in higher eukaryotes, namely a pulsatile synthesis and degradation used by Bacteria as a signal to control their aggregation properties as a differentiating multicellular organism.
The universal role of cAMP in controlling such diverse metabolic processes is puzzling because adenylyl cyclases are extremely varied, and submitted to a wide variety of regulations. Why does this result in the synthesis of the same molecular species, cAMP? Is not all the regulatory process lost in this way? How can the cAMP signal generated by one enzyme type be distinguished from another? Compartmentalization is often invoked in this process, but while this is relatively easily accounted for in the case of macromolecules, this is difficult to see in the case of small molecules such as cAMP. Another usual answer is to say that it is the combination of hormonal receptors of differing types and cAMP — and not cAMP alone — that is required for specificity. But would not cAMP synthesized from different sources also be recognized? Another answer is to remark that cAMP is known to be only one among many second messenger : cGMP has been added to the list as well as inositol phosphates, phosphatidyldiglycerides, calcium etc. This certainly permits generation of a combinatorial control of activities, but would certainly be very sensitive to accidental synthesis of cAMP. Cyclic AMP is not synthesized in a steady-state way (even in bacteria). It is therefore important to consider not cAMP as such, with some average concentration, but to consider the shape of its time-dependent variation in concentration. In fact observations are accumulating that strongly suggest that cAMP does not have the same effect when it is delivered in a steady state fashion, rather than in a pulse (or a series of pulses).
The motile and aggregating amoeba D. discoïdeum has been used as a paradigm for cell differentiation because undifferentiated cells start to differentiate into specific tissues some time after starvation. Secreted in the external medium cAMP is necessary for aggregation. The genetics, biochemistry, cellular biology and physiology of phenomena involving cAMP have been investigated in detail in this organism, where it controls, as in higher eukaryotes, a protein phosphorylation cascade, initiated after a regulatory cAMP-binding subunit of a protein kinase detaches from its target enzyme. This cascade is necessary not only for chemotaxis and aggregation but also for the triggering of genes involved in differentiation. The regulation of cAMP pulsatile concentration is mediated by two sets of enzymes, adenylate cyclases and phosphodiesterases. In contrast with the situation with higher eukaryotes however, cAMP and phosphodiesterase control operates not from the interior of the cell but from the external medium. This requires specific membrane receptors for cAMP, and a process of signal transduction. In D. discoïdeum the pulses are generated by an appropriate coupling between adenylate cyclase activity, phosphodiesterase activity and diffusion. The main observation is that variation in the cAMP pulse frequency changes the response of the cell. Many biochemical models can account for such cAMP pulses. These models require simple enzyme properties (in particular standard non linear features, such as self-activation, and desensitization after saturating activity). They do not require the existence of many gene products, but only a specific behaviour of enzymes (appropriate Vm and Km of biosynthetic and degradative enzymes). Assuming that cAMP concentration modulation in time is the control event is therefore not a biochemical paradox.
In Bacteria, Utsumi et al. have investigated cyclic AMP synthesis during the cell cycle of E.coli on synchronized cells, and they have given an unambiguous demonstration that there was a strong correlation of cAMP synthesis and replication or cell division, suggesting that the molecule may play some role in the cell cycle. This is also correlated with the position of the adenylate cyclase gene near the chromosome's origin of replication, and with its very low level of expression, suggesting that expression is strongly coupled to DNA replication. This observation has long been overlooked because cells deficient for adenylate cyclase or CAP are viable, suggesting that cAMP is dispensable. Specific time-dependent variation of the concentration of cAMP for fine coordination of replication and division in E. coli is achieved by excretion of the nucleotide, rather than coupling to the activity of a phosphodiesterase. In this respect it is interesting that high concentrations of cAMP produced by foreign genes in E. coli are not toxic, till they reach a very high level (at least 10-fold the normal concentration), whereas much lower concentration of cAMP produced by the endogeneous adenylate cyclase are toxic. Cyclic AMP has been formally linked to catabolite repression, but there exists many catabolite sensitive operons that are not responding to cAMP. In addition cAMP synthesis is very strong in E. coli when cells enter the stationary phase of growth, suggesting that it could be a cell to cell signal as it is in D. discoïdeum. This may be one of its function in other bacteria (such as Rhizobium species), where it is clearly not linked to catabolite repression.
In the same way, intracellular and extracellular levels of cAMP vary during the cell cycle of Saccharomyces cerevisiae. Using centrifugal elutriation, Smith et al. showed that the intracellular cAMP concentration followed the stages of the cell cycle, being highest during the division cycle and lowest immediately prior or just after cell separation; at the same time the external cAMP concentration did not vary. Therefore, in yeast as in E. coli it appears that the role of the external medium is to behave as a sink. These observations substantiate the demonstration that, under normal conditions, appropriate enzyme systems can generate a specific time-dependent pattern of cAMP concentration. As in the case of E. coli it is known that in S. cerevisiae adenylyl cyclase is dispensable in mutants of the cAMP receptor, and in S. pombe adenylate cyclase is dispensable during vegetative growth. But, as in this former case, cells that carry the mutation and are deficient in adenylate cyclase have several growth defects. In this respect the function of the time-dependent cAMP pattern could be optimisation of transient processes, in particular cell division and chromosome segregation.
In all these cases cAMP is recognized on the cell surface by a specific receptor. It is therefore interesting to identify cases where membrane targets of cAMP have been demonstrated (D. discoïdeum aside). Nerve cells typically generate and are sensitive to transient signals, they also have very invoved patterns of adenylate and guanylyl cyclases regulation. In this respect it is important to observe that cGMP (but also cAMP), has been shown to be involved as a central molecule in vision, taste and olfaction. In particular, in addition to their role as second messengers in protein phosphorylation cascades, cyclic nucleotides are involved at the membrane surface, but intracellularly, in gating ion channels in olfactory and taste neurons. This certainly permits generation of a variety of time-dependent patterns for cAMP regulation, as a function of environmental inputs as well as of the fine molecular structure of the enzyme or its subunits. Because ion channels are involved in the main functions of neurons (firing patterns) this makes cyclic nucleotides important in learning processes.
Indeed, many experiments have demonstrated that cAMP is involved as a mediator of learning and memory in invertebrates (Aplysia and Drosophila melanogaster), as well as in vertebrates. The study of mutants of D. melanogaster that are defective in learning and/or memory has been of major importance in our understanding of the physiology, biochemistry, and anatomy underlying conditioned behaviors. D. melanogaster learning mutants have been separated into two general classes, those with structural defects in the brain and those without obvious brain alterations. From studies of mutants affected in the brain structure, two areas have been found to be involved in conditioned behavior: the mushroom bodies and the central complex. Analysis of the mutants has shown that many types of molecules are involved in learning, but the cAMP-mediated phosphorylation cascade has emerged as especially important. During learning time-dependent processes are involved in the stabilization of synapses, a general view being that they are created during growth as transient entities which can either regress or be stabilized. In this process, the evolution of the synaptic pattern is dependent on the pattern of neurotransmitter delivery. Analysis of the minimal requirements for synapses stabilization suggests that neurotransmitter release must be coupled to some other transient metabolic process in a retrograde manner to yield a stable geometry. In the cases where cAMP is involved, one can therefore speculate that the role of this mediator is to trigger an appropriate biochemical process, when the proper time-dependent control of its synthesis is at work. Accordingly, once again, it is not the cAMP concentration which is important, but, rather, the time-variation of its concentration. In the process of learning the regulation of adenylate cyclase activity would therefore be exquisitely tuned to permit delivery of the molecule in the proper time-dependent manner.
In D. melanogaster, five different genes have proven important for normal learning: dunce (a cAMP phosophodiesterase), rutabaga (an adenylate cyclase), amnesiac (a product similar to adenylate cyclase activating peptides), DCO (protein kinase A), and dCREB2 (a cAMP-response element binding protein). The products of many of these learning mutants are enriched in mushroom bodies. A process involving control of transcription by the cAMP response element binding protein (CREB)-responsive plays a central role in the formation of long-term memory in D. melanogaster, Aplysia and mammals. This is one of the examples where cAMP is invoved in the control of transcription in Eukarya, as it is in Bacteria, although through a different chain of events. Agents that prevent CREB activity interfere with the formation of long-term memory, whereas agents that increase the amount or activity of the transcription factor accelerate the process, thus indicating that CREB is essential for the switch from short-term memory to long-term memory (protein synthesis dependent). Further work involving inbred mice strains as well as knock-out mutants affecting the hippocampal region demonstrated that both the genetic background and the temporal pattern of synaptic activity affects the cAMP-dependent synaptic plasticity.
Cyclic GMP is a structural analogue of cyclic AMP which occurs at similar low concentration (i.e. in the micromolar range) in many animal tissues. Its presence as a functional molecule in Bacteria is controversial. It has been shown to be present in E. coli, but the corresponding intracellular concentration, in the nanomolar range, makes it hardly a significant molecule (this would mean ca one molecule per cell). In addition the sequence of the genome did not reveal any polypeptide structure that could code for guanylate cyclase in this organism. There are many reports suggesting the presence of cGMP in Bacteria, but its presence is perhaps really likely in myxobacteria, where it could be involved in cell aggregation and differentiation. The recent discovery of the rather ubiquitous presence of cyclic di-guanosine-monophosphate might account for interference in dosage of guanylyl nucleotides. In contrast cGMP has been demonstrated universally in Eukarya (except in plants). It is generally involved in processes leading to activation of specific regulation cascades, differentially controlled by appropriate mediators, or to control specific processes of neuronal activation of sense organs, such as the sensitivity to light of retina receptor cells, and the triggering of olfaction and taste. The organisation of guanylyl cyclase and control elements is sometimes similar to — but distinct from — the organisation of hormonally regulated adenylate cyclase. In particular G-proteins mediated regulation of vision operates on phosphodiesterase rather than on cyclase.
Phototransduction systems in vertebrates and invertebrates share a great deal of similarity in overall strategy but differ significantly in the underlying molecular machinery. Vertebrate retinal rod cells, in the dark, synthesize a high level of cGMP that keeps open gated sodium channels in the plasma membrane of the outer segment. Light closes these channels by activating an enzymatic cascade that leads to rapid hydrolysis of cGMP by cGMP-specific phosphodiesterase. This hyperpolarizes the cell, and modulates transmitter release at the synaptic buttons. Photoexcited rhodopsin triggers a transducer protein (transducin, related to G-proteins) by catalyzing the exchange of GTP for bound GDP. Subsequently, the activated GTP-form of transducin switches phosphodiesterase on. The cascade (overall gain 105) is turned off by the GTPase activity of transducin and by the action of two proteins, rhodopsin kinase and arrestin. The kinetics of reactions in the cGMP cascade limit the temporal resolution of the visual system, while statistical fluctuations in the reactions limit the reliability of detection of dim light. Together with calcium ions,and inositol phosphates cGMP controls visual excitation and adaptation. A light-induced fall in the internal free Ca2+ concentration subsequently stimulates resynthesis of cGMP, antagonizes the catalytic activity of rhodopsin, and restores the high affinity of the light-regulated sodium channel for cGMP allowing the cell to adapt in background light. The initial events in molluscs and arthropods are probably similar to those of vertebrates. However, whereas light activation of vertebrate photoreceptors leads to activation of cGMP-phosphodiesterase and generation of a hyperpolarizing response, activation photoreceptors of invertebrate like Drosophila, leads to stimulation of phospholipase C and generation of a depolarizing response. Cyclic GMP has also been implicated in the modulation of behaviour in insects.
Cyclic GMP is also a secondary messenger in regulation mediated by natriuretic peptide hormones, but it is probably in the recently discovered nitric oxide (NO) regulation cascade, that cGMP has the most unexpected role. Nitric oxide and natriuretic peptide hormones (Atrial Natriuretic Peptides, ANPs) play key roles in a number of neuronal functions, including learning and memory as well as in the circulation of blood. Most experiments suggest that they exert converging actions by elevation of intracellular cGMP levels through activation of soluble and membrane bound guanylyl cyclases. Cyclic GMP is the starting point for multiple signaling cascades, which are now beginning to be unravelled. In more than a quarter of a century the discoveries of the existence of atrial granules and of volume receptors in the heart atria the search for natriuretic hormones has led to the isolation and identification of many atrial natriuretic factors (ANF). In the heart, for example, ANF peptides are synthesized and stored in the Golgi apparatus of cardiac myocytes, and are released in response to atrial wall stretch following acute plasma volume expansion and increased central blood volume. The mechanisms of the renal action of these potent natriuretic hormones are not yet completely unraveled. The renal hemodynamic and tubular as well as the adrenal and systemic vascular effects are related to enhanced cGMP synthesis in specific medium-sized arteria, as well as in the glomeruli and specific tubular segments, and in adrenal tissue. Specific ANF-binding sites have been detected in these target organs. A primary action of elevated cGMP levels is the stimulation of cGMP-dependent protein kinase (PKG), the major intracellular receptor protein for cGMP, which phosphorylates substrate proteins to trigger a regulation cascade.
Cyclic GMP-dependent protein kinases also mediate some of the neuronal effects of cGMP. Unfortunately few PKG substrates are known in the brain. In striatonigral nerve terminals, for example, NO mediates phosphorylation of the protein phosphatase regulator dopamine- and cyclic AMP-regulated phosphoprotein by PKG. It appears that PKG substrates are critically placed in the protein phosphorylation network and regulate protein phosphatases, intracellular calcium levels, and the function of many ion channels and neurotransmitter receptors. Nitric oxide acts as a signalling molecule in the nervous system of both mammals and insects. In contrast to classical transmitters, the NO permeates membranes, being able to act on neighbouring targets normally limited by diffusion barriers. This diffuse signalling is evolutionarily highly conserved. The NO forming enzyme, NO synthase, is mostly present in the nervous system, especially the brain. A soluble form of guanylyl cyclase, is the major target of NO action. Usually there is cellular separation of the release site and target site of NO, although exceptions to this rule exist. As cAMP, cGMP seems to be important for memory in insects : in the honeybee for exemple, the NO/cGMP system present in the antennal lobes is implicated in the processing of adaptive mechanisms during chemosensory processing, and experimental data support a specific role of the NO system in memory formation.
Signal transduction in gastric and intestinal smooth muscle is mediated by receptors coupled via distinct G proteins to various effector enzymes. Calcium is implicated in signal transdcution in different ways according to the cell type (e.g. circular and longitudinal muscle cells). The initial steps involve Ca2+/calmodulin-dependent activation of myosin light chain kinase and interaction of actin and myosin. Relaxation is mediated by cAMP-and/or cGMP-dependent protein kinase. A specific cascade involves G-protein-dependent stimulation of Ca2+ influx leading to Ca2+/calmodulin-dependent activation of a constitutive NO synthase in muscle cells, activating soluble guanylyl cyclase. The resultant activation of PKA and PKG is jointly responsible for muscle relaxation.
Cyclic GMP is therefore a secondary messenger acting on targets that are sometimes similar to the cAMP targets, but that proceeds through a completely different cascade, in which the diffusible NO (and perhaps sometimes CO, which is produced even by bacteria), plays a major role. The nitric oxide / cyclic GMP pathway has attained public celebrity when it was found that an inhibitor of a specific class of cGMP phosphodiesterase, involved in the control of vasodilatation required for penile erection could be selectively inhibited by a fairly innocuous compound, sildenafil (UK-92,480), known by its commercial name of the Viagra blue pill.
![]()
Octavian Barzu passed away during the night of 24-25 april 2010. He was instrumental in the deciphering of the biochemical properties of class II adenylate cyclases.
Trained as a chemist and as a physician he started to develop his research in Romania. Having difficulties to live in a country dominated by the dictatorship of Nicolae Ceauşescu he flew away to France where he first worked with Roger Monier in Marseilles, then Agnès Ullmann in Paris. He succeeded, later on, to get his family coming to join him as political emigrants. Recruited at the CNRS he could rapidly set up his own laboratory, devoted to the biochemical studies of nucleotide binding enzymes.
A strong personality he was always extremely precise and careful in the way he undertook and interpreted his experiments. Yet he accepted some fantasy, and this is how, being quite complementary, we could develop a collaboration that lasted for more than two decades. He was forced to compulsory retirement five years ago because of age limit, and had I not found room for him in my laboratory, he would have faced the unenviable fate of many scholars in France (we may remember Frank Kunst here), who are killed by the mediocrity of their obscure "peers" who are too happy to use an irrelevant law to get rid of persons who are standing in their (absence of) light. It can be expected, as usual, that those who made his life most difficult will now lament about his absence. Life is a cynical theater.
Octavian Barzu had a keen interest for motivated research of the type that Louis Pasteur initiated more than a century ago. And we had the fascinating experience of producing in the laboratory enzymes that could be used to solve bottlenecks in industry, that allowed companies to produce compounds at a low price. And it was amusing to find out, much later on , that some of our production was present in highly fashionable cosmetics, after a quite unexpected "travel".
His list of publications referenced at PubMed gives a good idea of the high quality of his work.
One of his works, where he showed that norleucine can replace most methionines in proteins, has important implications in terms of modern synthetic biology:
Gilles AM, Marlière P, Rose T, Sarfati R, Longin R, Meier A,
Fermandjian S, Monnot M, Cohen GN, Bârzu O
Conservative replacement of methionine by norleucine in Escherichia
coli adenylate kinase
J Biol Chem. 1988 263: 8204-8209.
In the domain of nucleotidyl cyclases he reported that Bordetella pertussis adenylate cyclase could be split into several domains, and that re-association of the catalytic and the calmodulin-binding peptides could restore full activity. This was reminiscent of the well-established "alpha-omega" complementation in beta-galactosidase that was used for cloning genes by myriads of investigators all over the world.
A very modern thinker, he developed more recently a systematic
investigation of production of fine chemicals by combining enzymes in
vivo and in vitro in a process that will soon further develop
considerably with synthetic biology. And we pay tribute to the quality
of his work and his person here.
![]()
Agnès Ullmann passed away on February 25, 2019 after a life dedicated to science and to the Institut Pasteur. Her contributions have been described in a vivid autobiography, which also recounts the way, typical of a John Le Carré novel, she was able to escape the Communist dictatorship in Hungary. She played a key role in the development of my own scientific career and I often feel like talking to her today, as we did many times in her office, but I can no longer do so. My only consolation is that she did not witness the invasion of Ukraine, nor the COVID-19 outbreak. In addition to her conceptual insight that was instrumental in discovering the concept of the transcription promoter, she was such a remarkable experimenter that her notebooks should remain a model for students. Our collaboration began after we had cloned the gene of adenylate cyclase from E. coli and had postulated an unexpected function of the cAMP-CAP complex in the modulation of transcription polarity in operons. I had proposed a conjecture on the role of the tagging of the first methionine in translated proteins and I was looking for experiments that could (in)validate the conjecture. As we discussed, it rapidly became apparent that she had already done this type of experiment a decade previously, with exactly the predicted outcome! She had recorded the experiments with such fine details that it was easy to carry on. This was also the beginning of our long collaboration on adenylate cyclases. I gave a glimpse of this exceptional talent in my tribute (in French) to her at the Pasteur Institute a few months after her death. Archiving well the experiment notebooks of scientists should be a duty for any scientific institute, but, unfortunately, one can fear that this is not the case, especially when limited intelligence is demanded from those who are recruited to the management of research structures.