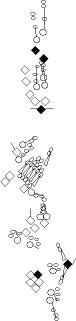
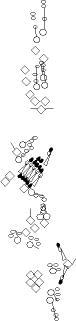
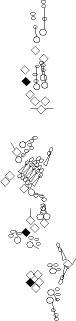
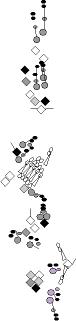
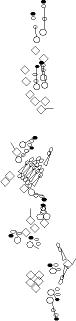
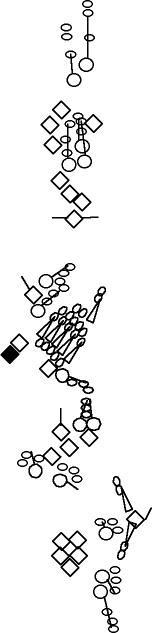
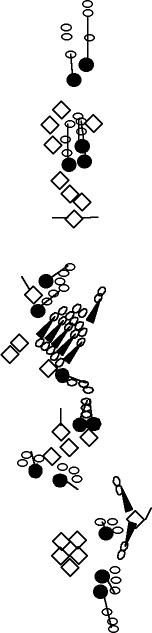
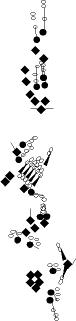
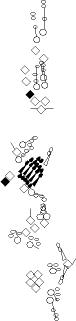
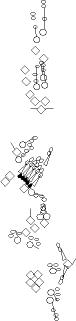
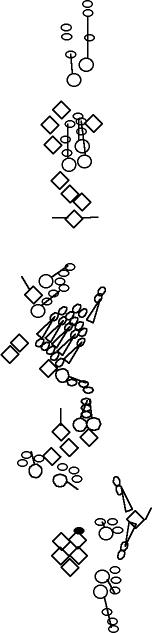
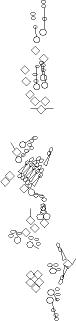
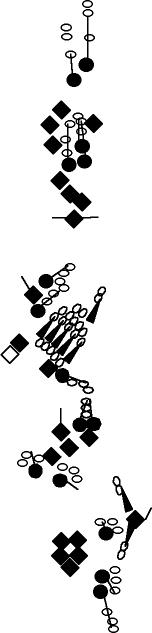
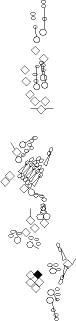
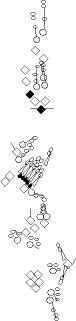
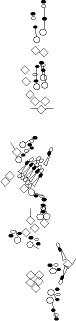
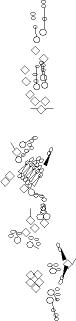
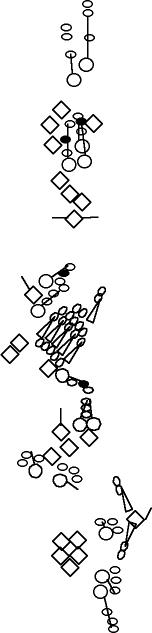
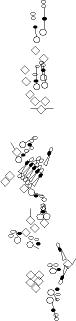
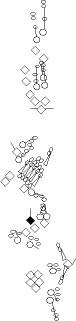
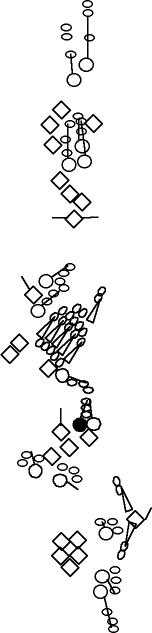
PNS expression patterns (1)
Antibodies
Expression
pattern in the embryonic
PNS at stage 15-17 is schematized here. Black cells are cells that
accumulate high levels of the marker, whereas grey cells accumulate low
levels and white cells do not accumulate the marker. Cells for which the expression has not been studied are represented by a light grey line. Expression pattern at earlier embryonic stages is also mentioned in the text below each diagram.
Click on a
marker name to link to the
corresponding FlyBase page.
|
|
Abrupt
|
|
alpha85E-tubulin
|
|
|
|
|
|
|
|
Abrupt is expressed in the class I neurons (vpda, ddaD and ddaE) as well as in vbd and dbd (Sugimura et al., 2004; Li et al., 2004).
|
|
alpha85E-tubulin is detected in cap cells, attachment cells and ligament cells of chordotonal organs (Matthews et al., 1990). It accumulates also in the ligament-attachment cell of the lch5 organ (not represented in the diagram) (Inbal et al., 2004). Rarely, two ligament-attachment cells are observed close to a lch5 organ (Inbal et al., 2004).
|
|
|
|
|
|
| Blimp-1
|
|
Collier = Knot
|
|
Cut
|
|
|
|
|
|
| blimp-1 is expressed in all the atonal-lacZ-expressing SOP cells (Ng et al., 2006), which will give rise to chordotonal organs. Blimp-1 protein and mRNA share the same temporal and spatial expression (Ng et al., 2006). |
|
described in Orgogozo et al., 2004. Same pattern as B6-2-25 and Pickpocket. This marker stains the class IV md neurons (Grueber et al., 2002). |
|
The Cut accumulation pattern has been primarily described in Blochinger et al., 1990. Its specific expression in multidendritic neurons is detailed in Grueber et al., 2003.
During earlier stages, Cut accumulates in primary precursor cells of
all external sensory organs and all the multidendritic neurons that
have been shown to originate from an md-es or md-solo lineage, as well as in their progeny
cells. Note that vbd
does not accumulate Cut at stage 16-17 but its precursor cells do
accumulate Cut (md-es lineage).
|
|
|
|
|
|
CYP303a1
|
|
|
|
|
|
|
|
|
|
|
This cytochrome P450 accumulates in the apical region of socket cells (Willingham and Keil, 2004).
|
|
|
|
|
|
|
|
|
|
Dimmed
|
|
RfX
|
|
Elav
|
|
|
|
|
|
|
Since the Gal4 enhancer-trap c929, which is inserted in the dimmed gene, is expressed in the lbd neuron (Hewes et al., 2003), Dimmed probably accumulates in the lbd neuron. Anti-Dimmed antibodies have been described in Allan et al. 2005 paper.
|
|
At
stage 15, Rfx/dRFX is found predominantly in nuclei of all chordotonal
(ch) and external sensory (es) organ neurons, and, at a lower level, in
the accessory sister cells resulting from the last asymmetric division
of the precursors (Vandaele et al., 2001). At stage 16 of
embryogenesis, Rfx progressively disappears in the accessory sister
cells and is only maintained in es and ch neuron nuclei at the end of
embryogenesis. |
|
Elav accumulates in all sensory neurons (Robinow and White, 1991).
Elav is also detected at a lower level in the vp1-vp4a lineages (pIIa,
pIIb, pIIIb cells) (V. Orgogozo and F. Schweisguth, personal observations).
Furthermore, at stage 16, a cortical staining is also observed
specifically in the sheath cell of all external sensory organs and in
the scolopale cell of all chordotonal organs with rat anti-Elav
antibodies from DSHB. This staining might not be related to Elav (V.
Orgogozo and F. Schweisguth, personal observations).
|
|
|
|
|
|
|
|
Erect wing
|
|
|
|
|
|
|
|
|
|
Erect wing accumulates in all the PNS neurons (DeSimone and White, 1993).
|
|
|
|
|
|
|
|
| Engrailed
|
|
Glial cells missing = Glide
|
|
|
|
|
|
|
|
|
| Engrailed accumulates faintly in all the lch5
cells (this expression pattern is probably related to the precursor
cells' originating from the posterior part of the segment), as well as
in dmd1 and lbd neurons (Brewster et al., 2001).
|
|
Glial
cells missing accumulates in ligament cells of chordotonal organs
and in glial cells originating from the CNS and PNS (Jones et al., 1995). One of the glial cells is associated with the dbd neuron (Fredieu and Mahowald, 1989). Same pattern as Repo and Wrapper. |
|
|
|
|
|
|
|
| Gooseberry-neuro |
|
Hamlet |
|
Krüppel |
|
|
|
|
|
|
| Gooseberry neuro accumulates in a single cell in the ventral epidermis (Gutjahr et al., 1993) that is located near the vbd neuron (V. Orgogozo and A. Moore, personal observations). This cell may thus be a glial cell associated to vbd. |
|
Hamlet
accumulation disappears at stage 15. It is expressed in the PNS
from stage 11 through stage 15. Within the md-es lineage, Hamlet
starts to accumulate in pIIIb cell and its progeny cells (neuron and sheath
cell). Hamlet quickly disappears in the sheath cell and continues to be
expressed by the differentiating neuron up to stage 15 (Moore et al., 2002). |
|
Krüppel is not detected in the PNS at stage 15-17. It is expressed in the PNS from stage 11 through stage 15. In the ventral region, Krüppel
is detected only in the vp1, vp2 and vp4a lineages and not in the
vmd1a, vp3 and vp4a lineages (vp5 not analyzed). In these three
lineages, Krüppel
accumulates in pIIa, pIIb, pIIIb and the multidendritic neuron (V.
Orgogozo and F. Schweisguth, personal observations). |
|
|
|
|
|
Kruppel homolog 1
|
|
Ladybird
|
|
Nubbin = Pdm1
|
|
|
|
|
|
|
Beck et al. 2004
paper indicates that Kruppel homolog 1 accumulates in all the PNS
neurons. However, there is one exception: it does not accumulates
in the lbd neuron (V. Orgogozo and A.W. Moore, personal observations).
|
|
Ladybird accumulates specifically in the vbd neuron (De Graeve et al., 2004).
|
|
Nubbin accumulates in dbd and its sibling glial cell, dmd1 and the ligament cells of chordotonal organs (Brewster et al., 2001). At stage 12-14, Nubbin accumulates at a low level in the primary precursor cells of the dbd and dmd1 neurons, and at a higher level in their daughter cells just after division (Umesono et al., 2002).
|
|
|
|
|
|
Onecut
|
|
Pax2 = Shaven = Sparkling
|
|
Paired
|
|
|
|
|
|
|
Onecut accumulates in PNS cells at stage 15 (Nguyen et al., 2000).
According to their position, the Onecut-expressing cells are probably
PNS neurons.
|
|
Pax2 accumulates in the shaft and sheath cells of all external sensory organs (Kavaler et al., 1999; Moore et al., 2004).
The exact Pax2 accumulation pattern in chordotonal organs and multidendritic
neurons has not been investigated. During md-es lineages, Pax2
accumulates at a low level in primary precursor cells and their progeny
cells pIIa, pIIb and pIIIb, in addition to the socket and sheath cells (Moore et al., 2004). |
|
Paired accumulates only in the neurons of vch1, vch2 and lch1. It is not detected in the lch5 neurons (V. Orgogozo and F. Schweisguth, personal observations).
|
|
|
|
|
|
|
|
peptidylglycine-alpha-hydroxylating monooxygenase (Phm)
|
|
|
|
|
|
|
|
|
|
Phm accumulates in the lbd neuron (Hewes et al., 2003).
|
|
|
|
|
|
|
|
| Pickpocket = mdNaC1 |
|
Pox neuro
|
|
Pdm2/Miti-mere
|
|
|
|
|
|
|
| described in Adams et al., 1998. Same pattern as B6-2-25 and Collier. This marker stains the class IV md neurons (Grueber et al., 2002). |
|
Pox-neuron accumulates in a cell closely associated with each of the four hair es organs dh1, dh2, lh1 and lh2 (Awasaki and Kimura, 2001). This cell is presumably the hair-secreting shaft cell. In poxn mutants, hairs are replaced by papilla-like structures (Awasaki and Kimura, 2001).
|
|
According to in situ stainings, pdm2 is expressed in the same embryonic PNS cells as nubbin/pdm1 (Llyod et al., 1991; Dick et al., 1991). Anti-Pdm2 antibodies are described in Yeo et al., 1995.
|
|
|
|
|
|
|
|
Pyrexia
|
|
Prospero
|
|
|
|
|
|
|
|
Pyrexia accumulates in multidendritic neurons as well as in non-multidendritic neurons in larval epidermis (Lee et al., 2005) but its specific expression pattern has not been characterized.
|
|
Prospero accumulates in the sheath cell of all external sensory organs as well as all scolopale cells of chordotonal organs (Doe et al., 1991; Vaessin et al., 1991).
|
|
|
|
|
|
| Repo = Reverse Polarity
|
|
Runt
|
|
Senseless
|
|
|
|
|
|
|
| Repo accumulates in ligament cells of chordotonal organs and in glial cells originating from the CNS and PNS (Campbell et al., 1994; Halter et al., 1995; Xiong et al., 1994; Umesono et al., 2002). One of the glial cells is associated with the dbd neuron (Fredieu and Mahowald, 1989). Same pattern as Glial cells missing and Wrapper.
|
|
Runt only accumulates in one sensory cell in the ventral region, the socket cell or the shaft cell of the vp3
organ (undetermined, V. Orgogozo and F. Schweisguth, personal observations). Runt
accumulation pattern in the lateral and dorsal regions has not been
investigated.
|
|
At stage 15-17, Senseless only accumulates in the vtd2 neuron (V. Orgogozo, personal observations). It also accumulates in a subepidermal cell located near vp5, which
is part of a three-cell epidermal gland (V. Orgogozo and F. Schweisguth, unpublished
results). At stage 10-14, Senseless accumulates in the primary
precursor cells of external sensory organs, chordotonal organs and
multidendritic neuron (Nolo et al., 2000). The staining then decreases gradually in the progeny cells and is lost first in the pIIa progeny cells .
|
|
|
|
|
|
| Sequoia
|
|
Sloppy paired |
|
Spineless
|
|
|
|
|
|
|
| At stage 14, Sequoia is detected in nuclei of neuron and sheath cells but not the socket and shaft cells (Brenman et al., 2001).
By late stage 16, Sequoia is most abundant in neurons but not
detectable in sheath cells. Sequoia accumulates in all the
Elav-positive neurons of the drosal region at stage 16.
|
|
Sloppy-paired
is not detected in the ventral PNS at stage 15-17. It is
expressed in the PNS from stage 11 through stage 15. In the ventral
region, Sloppy-paired accumulates only in the vp1, vp2, vp4 and vp4a
lineages but not in the vp3 lineage (vp5 not analyzed). In these
lineages, it accumulates at a high level in the pI cell, decreases in
the pIIa-pIIb cell pair and then disappears (V. Orgogozo and F. Schweisguth, personal
observations). |
|
Spineless accumulates in all the PNS neurons (Duncan et al., 1998; Kim et al., 2006).
|
|
|
|
|
|
|
|
Suppressor of Hairless
|
|
target of Poxn
|
|
|
|
|
|
|
|
|
Suppressor of Hairless accumulates at a high level in the socket cell of all external sensory organs (Gho et al., 1996; Barolo et al., 2000).
A GFP reporter gene under the control of a Suppressor of Hairless
regulatory region is also very specifically expressed in all external
sensory organ socket cells (Barolo et al., 2000). |
|
Target of Poxn accumulates only in the anterior es neuron of the bi-innervated vp5 organ (Gautier et al., 1997). Expression appears once all the sensory cells are formed.
|
|
|
|
|
|
| Wrapper
|
|
|
|
|
|
|
|
|
|
|
| Wrapper accumulates in ligament cells of chordotonal organs and in glial cells originating from the CNS and PNS (Noordermeer et al., 1998). One of the glial cells is associated with the dbd neuron (Fredieu and Mahowald, 1989). Same pattern as Glial cells missing and Repo. |
|
|
|
|
|
|
|
|
|
References
Adams, C.M., Anderson, M.G.,
Motto, D.G., Price, M.P., Johnson, W.A. and Welsh, M.J., 1998. Ripped
pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in
early development and in mechanosensory neurons. J Cell Biol. 140, pp. 143-52. Medline
Allan DW, Park D, St Pierre SE, Taghert PH, Thor
S. 2005. Regulators acting in combinatorial codes also act
independently in single differentiating neurons. Neuron. Mar
3;45(5):689-700. Medline
Awasaki T, Kimura K. 2001. Multiple function of
poxn gene in larval PNS development and in adult appendage formation of
Drosophila. Dev Genes Evol. 211(1):20-9. Medline
Barolo, S., Walker, R., Polyanovsky, A., Freschi, G., Keil, T. and Posakony, J. W.,
2000. A Notch-independent activity of Suppressor of Hairless is
required for normal mechanoreceptor physiology. Cell 103, pp. 957-969. Medline
Beck Y, Pecasse F, Richards G. 2004.
Kruppel-homolog is essential for the coordination of regulatory gene
hierarchies in early Drosophila development. Dev Biol. 268(1):64-75. Medline
Blochlinger,
K., Bodmer, R., Jan, L.Y. and Jan, Y.N., 1990. Patterns of expression
of Cut, a protein required for external sensory organ development, in
wild-type and cut mutant Drosophila embryos. Genes
Dev. 4, pp. 1322-1331. Medline
Blochlinger,
K., Jan, L.Y. and Jan, Y.N., 1991. Transformation of sensory organ
identity by ectopic expression of Cut in Drosophila. Genes
Dev. 5, pp. 1124-1135.
Medline
Brenman JE, Gao FB, Jan LY, Jan YN. 2001. Sequoia, a tramtrack-related zinc finger protein, functions as a
pan-neural regulator for dendrite and axon morphogenesis in Drosophila. Dev Cell. 1(5):667-77. Medline
Brewster,
R. and Bodmer, R., 1995. Origin and specification of type II sensory
neurons in Drosophila. Development 121,
pp. 2923-2936. Medline
Brewster, R., Hardiman, K., Deo, M., Khan, S. and Bodmer, R., 2001. The selector gene cut represses
a neural cell fate that is specified independently of the
Achaete-Scute-Complex and atonal. Mech Dev. 105, pp. 57-68. Medline
Campbell,
G., Goring,
H., Lin, T., Spana, E., Andersson, S., Doe, C.Q. and Tomlinson, A.,
1994. RK2, a glial-specific homeodomain protein required for embryonic
nerve cord condensation and viability in Drosophila. Development
120, pp. 2957-2966. Medline
De Graeve, F., Ja, T., Daponte, J.-P.,
Rickert, C., Dastugue, B., Urban, J. and Jagla, K.. 2004. The ladybird
homeobox genes are essential for the specification of a subpopulation
of neural cells. Dev Biol 270, pp. 122-134. Medline
DeSimone SM, White K. 1993. The Drosophila erect wing gene, which is important for both
neuronal and muscle development, encodes a protein which is similar to
the sea urchin P3A2 DNA binding protein. Mol Cell Biol. 13(6):3641-9. Medline
Dick, T., X Yang, S Yeo, and W Chia. 1991. Two closely linked Drosophila POU domain genes are expressed in neuroblasts and sensory elements. Proc Natl Acad Sci U S A. 88, pp. 7645-9. Medline
Doe,
C.Q., Chu-LaGraff, Q., Wright, D.M. and Scott, M.P., 1991. The prospero
gene specifies cell fates in the Drosophila central nervous
system. Cell 65, pp. 451-464. Medline
Duncan DM, Burgess EA, Duncan I. 1998. Control of distal antennal identity and tarsal development in
Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin
receptor.
Genes Dev. 1998 May 1;12(9):1290-303. Medline
Fredieu, J.R and Mahowald, A.P., 1989. Glial interactions with neurons during Drosophila embryogenesis. Development 106, pp. 739-48. Medline
Gautier P, Ledent V, Massaer M, Dambly-Chaudiere
C, Ghysen A. 1997. tap, a Drosophila bHLH gene expressed in
chemosensory organs. Gene. 1997 May 20;191(1):15-21. Medline
Gho, M., Lecourtois, M., Geraud, G., Posakony,
J.W., and Schweisguth, F. 1996. Subcellular localization of
Suppressor of Hairless in Drosophila sense organ cells during Notch
signalling. Development 122, 1673-1682. Medline
Grueber,
W.B., Jan, L.Y. and Jan, Y.N., 2002. Tiling of the Drosophila
epidermis by multidendritic sensory neurons. Development 129,
pp. 2867-2878. Medline
Grueber, W. B., Jan, L. Y. and Jan, Y. N.
2003. Different levels of the homeodomain protein cut regulate distinct
dendrite branching patterns of Drosophila multidendritic neurons. Cell
112, pp. 805-18. Medline
Gutjahr T, Patel NH, Li X, Goodman CS, Noll M. 1993. Analysis of the gooseberry locus in Drosophila embryos: gooseberry
determines the cuticular pattern and activates gooseberry neuro. Development. 1993 May;118(1):21-31. Medline
Halter, D.A., Urban, J., Rickert, C., Ner, S.S.,
Ito, K., Travers, A.A. and Technau, G.M., 1995. The homeobox gene repo
is required for the differentiation and maintenance of glia function in
the embryonic nervous system of Drosophila melanogaster. Development 121, pp. 317-32. Medline
Hartenstein,
V. and Jan, Y.N., 1992. Studying Drosophila embryogenesis with
P-lacZ enhancer trap lines. Roux's Arch. Dev. Biol. 201,
pp. 194-220.
Hewes RS, Park D, Gauthier SA, Schaefer AM,
Taghert PH. 2003. The bHLH protein Dimmed controls neuroendocrine cell
differentiation in Drosophila. Development 130(9):1771-81. Medline
Inbal, A., Volk, T. and Salzberg, A. 2004.
Recruitment of ectodermal
attachment cells via an EGFR-dependent mechanism during the
organogenesis of Drosophila proprioceptors. Dev Cell. 7, pp.241-50. Medline
Jones, B.W., Fetter, R.D., Tear, G. and
Goodman, C.S., 1995. glial cells missing: a genetic switch that
controls glial versus neuronal fate. Cell 82, pp. 1013-23. Medline
Kavaler J, Fu W, Duan H, Noll M, Posakony JW.
1999. An essential role for the Drosophila Pax2 homolog in the
differentiation of adult sensory organs. Development 126, pp. 2261-72. Medline
Kim MD, Jan LY, Jan YN. 2006. The bHLH-PAS protein Spineless is necessary for the diversification
of dendrite morphology of Drosophila dendritic arborization neurons. Genes Dev. 20(20):2806-19. Medline
Lee Y., Lee Y., Lee J., Bang S., Hyun S., Kang J.,
Hong S.T., Bae E., Kaang B.K., Kim J. 2005. Pyrexia is a new thermal
transient receptor potential channel endowing tolerance to high
temperatures in Drosophila melanogaster. Nat Genet. 37, pp. 305-10. Medline
Li W, Wang F, Menut L, Gao FB. 2004.
BTB/POZ-Zinc Finger Protein Abrupt Suppresses Dendritic Branching in a
Neuronal Subtype-Specific and Dosage-Dependent Manner. Neuron 43, pp.
823-34. Medline
Lloyd A, Sakonju S. 1991. Characterization of two Drosophila POU domain genes,
related to oct-1 and oct-2, and the regulation of their expression
patterns. Mech Dev. 36, pp. 87-102. Medline
Matthews,
K.A.,
Miller, D.F. and Kaufman, T.C., 1990. Functional implications of the
unusual spatial distribution of a minor alpha-tubulin isotype in Drosophila:
a common thread among chordotonal ligaments, developing muscle, and
testis cyst cells. Dev. Biol. 137, pp.
171-183. Medline
Moore, A.W., Jan, L.Y. and Jan, Y.N., 2002.
hamlet, a binary genetic switch between single- and multiple- dendrite
neuron morphology. Science 297, pp. 1355-8. Medline
Moore, A.W., Roegiers, F., Jan, L.Y. and Jan,
Y.N., 2004. Conversion of neurons and glia to external-cell fates in
the external sensory organs of Drosophila hamlet mutants by a
cousin-cousin cell-type respecification. Genes Dev. 18, pp. 623-8. Medline
Ng T, Yu F, Roy S. 2006. A homologue of the vertebrate SET domain and zinc finger protein
Blimp-1 regulates terminal differentiation of the tracheal system in
the Drosophila embryo. Dev Genes Evol. 216(5):243-52. Medline
Nguyen DN, Rohrbaugh M, Lai Z. 2000. The Drosophila homolog of Onecut homeodomain proteins is a
neural-specific transcriptional activator with a potential role in
regulating neural differentiation. Medline
Nolo R, Abbott LA, Bellen HJ., 2000.
Senseless, a Zn finger transcription factor, is necessary and
sufficient for sensory organ development in Drosophila. Cell 102, pp. 349-62. Medline
Noordermeer,
J.N.,
Kopczynski, C.C., Fetter, R.D., Bland, K.S., Chen, W.Y. and Goodman,
C.S., 1998. Wrapper, a novel member of the Ig superfamily, is expressed
by midline glia and is required for them to ensheath commissural axons
in Drosophila. Neuron 21, pp.
991-1001. Medline
Orgogozo, V., Schweisguth, F. and Bellaiche, Y. 2004. Slit-Robo signalling prevents sensory cells from crossing the midline in Drosophila. Mech Dev. 121, pp. 427-36. Medline
Robinow, S. and White, K., 1991.
Characterization and spatial distribution of the ELAV protein during
Drosophila melanogaster development. J Neurobiol. 22, pp. 443-61. Medline
Sugimura, K., Satoh, D., Estes, P., Crews, S.
and Uemura, T. 2004. Development of Morphological Diversity of
Dendrites in Drosophila by the BTB-Zinc Finger Protein Abrupt. Neuron 43, pp. 809-22. Medline
Umesono,
Y., Hiromi, Y. and Hotta, Y., 2002. Context-dependent utilization of
Notch activity in Drosophila glial determination. Development
129, pp. 2391-2399. Medline
Vaessin,
H., Grell,
E., Wolff, E., Bier, E., Jan, L.Y. and Jan, Y.N., 1991. prospero is
expressed in neuronal precursors and encodes a nuclear protein that is
involved in the control of axonal outgrowth in Drosophila. Cell
67, pp. 941-953. Medline
Vandaele C, Coulon-Bublex M, Couble P, Durand
B. 2001. Drosophila regulatory factor X is an embryonic type I sensory
neuron marker also expressed in spermatids and in the brain of
Drosophila. Mech Dev. 103(1-2):159-62. Medline
Willingham, A.T. and Keil, T., 2004. A tissue
specific cytochrome P450 required for the structure and function of
Drosophila sensory organs. Mech Dev. 121, pp. 1289-1297. Medline
Xiong,
W.C., Okano,
H., Patel, N.H., Blendy, J.A. and Montell, C., 1994. repo encodes a
glial-specific homeo domain protein required in the Drosophila
nervous system. Genes Dev. 8, pp. 981-994. Medline
Yeo SL, Lloyd A, Kozak K, Dinh A, Dick T, Yang X, Sakonju S. and Chia W., 1995. On the functional overlap between two
Drosophila POU homeo domain genes and the cell fate specification of a
CNS neural precursor. Genes Dev. 9, pp. 1223-1236. Medline
zur Lage, P.I., Powell, L.M., Prentice, D.R.A.,
McLaughlin, P.M. and Jarman, A.P., 2004. EGF receptor signaling
triggers recruitment of Drosophila sense organ precursors by stimulating proneural gene autoregulation. Dev Cell 7, pp. 687-696. Medline
Comments and additions are welcome at: Virginie.Orgogozo snv.jussieu.fr