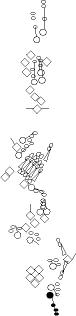
Sensory organ description
One can distinguish 3 types of sensory organs in
segments A1-A7
(Jan
and
Jan, 1993):
external sensory (es) organs which have external sensory structures
that detect mechanical or chemical signals, chordotonal (ch) organs
which are internal tube-shaped tenso-receptor structures located within
the
body wall, and multidendritic (md) neurons whose sensory modalities are
mainly unknown (prioproreceptors, thermoreceptors, nociceptors,
osmoreceptors, neurosecretory...). Multidendritic neurons are composed
of only one cell (the neuron) whereas es and ch organs comprise one or
several neurons associated with several
accessory cells. The neurons
innervating es and ch organs project a single dendrite containing a
modified cilium and are named type I neurons. Multidendritic neurons
are unciliated and project several dendrites, they are called type II
neurons. The multidendritic neurons are subdivided into the
dendritic arborization (da) neurons, the tracheal dendrite (td)
neurons, and the bipolar dendrite (bp) neurons. The da neurons have
been further classified into four different classes (I, II, III and IV)
based on dendrite morphology (
Grueber et al., 2002).
Chordotonal organs are
generally composed of several arrayed subunits called scolopidia, each
one comprising neuron(s) and accessory cells.
Despite this classification in three main
sensory types (external sensory organ, chordotonal organ and
multidendritic neuron), careful examination indicates that each
abdominal
sensory organ exhibits particular features.
Note also that there is no clear correlation between
the cell
lineage from which a sensory organ originates and its late morphology.
For example, the very similar class IV multidendritic neurons
v'ada and
vdaB derive from an
md-es lineage and an md-solo lineage respectively. Conversely, the very
different
vdaB and
vmd3/vbd
neurons come from similar md-es lineages.
You can click on the diagramm on a specific sensory organ to get its detailed
description (this tool does not work with Safari web browser).
|
|
|
vp3 = p3 = vc3 (vesC neuron) |
|
sensory
organ type |
ventral mono-innervated papilla (es organ) (four cells:
socket cell, shaft cell, sheath cell, neuron) |
|
morphology
|
papilla (Dambly-Chaudiere
and Ghysen, 1986). Unlike other abdominal socket cells, the vp3
socket
cell emits numerous short cytoplasmic extensions visible with the Su(H)-GFP transgene
described in Barolo et al., 2000
(V. Orgogozo, personal observations). However, no cuticular
particularity has been documented (Dambly-Chaudiere
and Ghysen, 1986). The unique characteristic of the vp3 socket cell
may be related to
the specific accumulation of Runt
in one of the vp3 accessory cells
(socket or shaft cells, undetermined) and in no other PNS cells (V.
Orgogozo, personal observations). |
|
development |
md-es lineage. Its md neuron associated by lineage is vbd (Orgogozo
et al., 2001). achaete-scute is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). |
|
|
|
vch1 = vchA (vchA neuron) |
|
sensory
organ type |
ventral mono-innervated chordotonal organ composed
of a single scolopidium. |
|
morphology
|
This scolopidium comprises three cells: cap
cell, scolopale cell and neuron. |
|
development |
not described.
atonal
is required for its formation (Jarman et al., 1993).
In spitz, rhomboid, pointed or Star
mutants, vch1 is absent whereas vch2
is still present (Okabe and Okano, 1997; zur Lage et al., 1997). |
|
|
|
vch2 = vchB (vchB neuron) |
|
sensory
organ type |
ventral mono-innervated chordotonal organ composed
of a single
scolopidium. |
|
morphology
|
This scolopidium comprises three cells: cap cell,
scolopale cell and neuron. |
|
development |
not described.
atonal
is required for its formation (Jarman
et al., 1993). In spitz,
rhomboid, pointed or Star mutants, vch2 is still
present whereas vch1 is still present (Okabe and Okano, 1997; zur
Lage et al., 1997). |
|
|
|
vdaB = vmd1a (one
of the vmd5 neurons) |
|
sensory
organ type |
ventral md neuron |
|
morphology
|
It presents a highly complex dendritic arborization and thus
belongs to
class
IV md neurons together with v'ada and ddaC (Grueber
et al., 2002). These three md neurons specifically accumulate the
following markers: Pickpocket
(Adams et al., 1998), Collier (Orgogozo
et al., 2004) and B6-2-25
(V. Orgogozo,
personal observations). |
|
development |
md-solo lineage (Orgogozo
et al., 2002).
Its primary precursor cell is located in the anterior part of the
abdominal segment as the primary precursor cell of the vp4
organ and v'ada neuron. In hamlet
mutant, the vdaB neuron is not duplicated whereas the other ventral md
neurons (originating from md-es lineages) are duplicated (W.
Grueber et al., 2003b, W. Grueber,
personal observations). This
suggests that in hamlet
mutants, the md neurons originating from an
md-solo lineage are lost whereas the ones originating from an md-es
lineage are duplicated. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). |
|
|
|
v'ada = vmd4a = vdaa |
|
sensory
organ type |
ventral md neuron |
|
morphology
|
It presents a highly complex dendritic arborization and thus
belongs to
class
IV md neurons together with vdaB and ddaC (Grueber
et al., 2002). These three md neurons specifically accumulate the
following markers: Pickpocket
(Adams et al., 1998), Collier (Orgogozo
et al., 2003) and B6-2-25
(V. Orgogozo,
personal observations). |
|
development |
md-es lineage. Its es organ associated by lineage is vp4a (Orgogozo
et al., 2001).
Its primary precursor cell is located in the anterior part of the
abdominal segment, as the vdaB's primary precursor
cell. achaete-scute is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). In hamlet
mutant MARCM clones, the v'ada neuron is duplicated (W.
Grueber et al., 2003b) (see vdaB). |
|
|
|
v'dap =v'pda |
|
sensory
organ type |
ventral md neuron |
|
morphology
|
Its
dendritic arborization harbors long primary and secondary
branches. Its dendrites have spiked protusions (1-20um long) along
almost their length. It
thus belongs to class III md neurons together with vdaD, ldaB,
ddaA and ddaF (Grueber
et al., 2002). Like them, it accumulates higher amounts of Cut than
other md neurons (Grueber et al., 2003). |
|
development |
not described.
achaete-scute is required for its formation (Dambly-Chaudiere
and Ghysen, 1987). Since
it is always found adjacent to both vp5 neurons, it
may be associated
by lineage to vp5. |
|
|
|
vtd1 (one of the v'td2 neurons) |
|
sensory
organ type |
ventral tracheal md neuron |
|
morphology
|
Its dendrites are associated to the trachea (Bodmer
and Jan, 1987). As opposed to other abdominal sensory neurons, vtd1
and vtd2 axons do not project within the segment
where they originate
but project more anteriorly up to segment T3 where they cross the
midline (Merritt and Whitington, 1995). |
|
development |
not described.
atonal is required for its formation (Jarman
et al., 1993). In spitz1
mutant, when both vch1 and vch2
neurons are absent (13/29 hemisegments), vtd1 and vt2 are always absent, and when one vch1/2
is still present (16/29), a single vtd neuron is sometimes present
(5/16) (V. Orgogozo, personal observations). This correlation suggests
that the vch cells, as well as Spitz/EGF signalling, are necessary for
vtd formation. BrdU
accumulation has been observed in vtd1 and vt2 (Bodmer
et al., 1989). |
|
|
|
vtd2 (one of the
v'td2 neurons) |
|
sensory
organ type |
ventral tracheal md neuron |
|
morphology
|
Its dendrites are associated to the trachea (Bodmer
and Jan, 1987).
As opposed to other abdominal sensory neurons, vtd1
and vtd2 axons do
not project within the segment where they originate but project more
anteriorly up to segment T3 where they cross the midline (Merritt
and Whitington, 1995). It is the only abdominal sensory cell to
accumulate Senseless at
stage 15-17 (V. Orgogozo, personal observations). |
|
development |
atonal is required
for its formation (Jarman
et al., 1993). In spitz1
mutant, when both vch1 and vch2
neurons are absent (13/29 hemisegments), vtd1 and vt2 are always absent, and when one vch1/2
is still present (16/29), a single vtd neuron is sometimes present
(5/16) (V. Orgogozo, personal observations). This correlation suggests
that the vch cells, as well as Spitz/EGF signalling, are necessary for
vtd formation. BrdU
accumulation has been observed in vtd1 and vt2 (Bodmer
et al., 1989). |
|
|
|
lh2
= b = shC = h1 (lesA neuron) |
|
sensory
organ type |
lateral mono-innervated hair (es organ) (four cells:
socket cell, shaft cell, sheath cell, neuron) |
|
morphology
|
hair
pointing towards the dorsal region (Dambly-Chaudiere
and Ghysen, 1986) |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby ldaA neuron. |
|
|
|
lp2
= p7 = lc1 (lesB neuron) |
|
sensory
organ type |
lateral mono-innervated papilla (es organ) (four cells:
socket cell, shaft cell, sheath cell, neuron) |
|
morphology
|
papilla (Dambly-Chaudiere
and Ghysen, 1986) |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby ltd neuron. |
|
|
|
lh1 = H = shC = h2 (lesC neuron) |
|
sensory
organ type |
lateral mono-innervated hair (es organ) (four cells:
socket cell, shaft cell, sheath cell, neuron) |
|
morphology
|
hair
pointing towards the dorsal region (Dambly-Chaudiere
and Ghysen, 1986) |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby ldaB neuron. |
|
|
|
lch5 (lch5 neurons) |
|
sensory
organ type |
lateral chordotonal organ composed
of five
scolopidia arrayed along the anterior-posterior axis. |
|
morphology
|
The two anterior scolopidia comprise five cells (from
posterior-dorsal to
anterior-ventral: attachment
cell, cap
cell,
scolopale cell, neuron and ligament cell) whereas the three posterior
scolopidia are deprived of an attachment cell and thus comprise four
cells. The ligament cells are also anchored to the cuticle through
other
specialized attachment cells (not represented in the diagram) (Inbal et al., 2004).
Note that there are two types of attachment cells: the lineage-related
ligament-attachment cells and the non-lineage-related cap-attachment
cells. All the cells of this sensory organ are the only abdominal
chordotonal cells to accumulate Engrailed (Brewster
et al., 2001). |
|
development |
Each scolopidium originates from a single primary precursor
cell (pI) (reviewed in Gould et al., 2001).
Three atonal-positive pI
cells appear first in the lateral region. They produce the three
anterior scolopidia through a yet undescribed lineage (see Lai and Orgogozo, 2004 for a probable cell
lineage). The most ventral pI cell also recruits two other ch pI cells
via
EGF/Spitz signalling. These two pI cells will form the two posterior
scolopidia through a yet undescribed lineage (this lineage is probably
the one that has been first proposed in Brewster et
al., 1995, see Lai
and Orgogozo, 2004 for details).
In spitz,
rhomboid, pointed or Star mutants, at least the
two posterior scolopidia are absent (Rutledge et
al.,
1992; Okabe and Okano, 1997; zur Lage et al., 1997).
In atonal
mutants, a single sclopidium is still formed at this position (Jarman
et al., 1993).
The most dorsal ch pI cell also induces the formation of six
neighboring oenocytes via
EGF/Spitz signalling. The difference in the fate induced by Spitz
(oenocytes versus ch pI cell) is determined by the Spalt expression
domain (Elstob et al., 2001; Rusten
et al., 2001).
During stages 12 -13, chordotonal cells migrate ventrally and
the whole chordotonal organ rotates while the attachment cells do not
change their position. Once the lch5 organ acquires the right
orientation, the ligament cells stretch ventrally (Inbal
et al., 2003),
and when they reach their final position in the lateral region,
ligament cells recruit attachment cells from the ectoderm through
EGF/Spitz signalling and induce their specialization (Inbal
et al., 2004). |
|
|
|
lch1 = lch1x = v'ch1 (v'ch1 neuron) |
|
sensory
organ type |
lateral chordotonal organ composed
of a single
scolopidium. |
|
morphology
|
This scolopidium comprises three cells: cap
cell, scolopale cell and neuron. |
|
development |
not described.
atonal
is required for its formation (Jarman
et al., 1993). spitz is
not required for its formation (Okabe
and Okano, 1997; zur Lage et al., 1997). |
|
|
|
ldaA = lda |
|
sensory
organ type |
lateral md neuron |
|
morphology
|
Its dendrites are simply branched but they are typically more
symmetrically bifurcating than the class I neurons. It thus
belongs to class II
md neurons together with vdaC, vdaA
and ddaB (Grueber
et al., 2002). |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby lh2
organ. |
|
|
|
ldaB |
|
sensory
organ type |
lateral md neuron |
|
morphology
|
Its
dendritic arborization harbors long primary and secondary
branches. Its dendrites have spiked protusions (1-20um long) along
almost their length. It
thus belongs to class III md neurons together with vdaD,
v'dap, ddaA and ddaF (Grueber
et al., 2002). Like them, it accumulates higher amounts of Cut than
other md neurons (Grueber et al., 2003). |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby lh1
organ. |
|
|
|
ltd = istd |
|
sensory
organ type |
lateral tracheal md neuron |
|
morphology
|
Its dendrites are associated with the trachea (Bodmer
and Jan, 1987). |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby lp2
organ. |
|
|
|
lbd = isbp = ldb |
|
sensory
organ type |
lateral bipolar md neuron |
|
morphology
|
It emits two long dendritic branches along muscle 8 (along
the dorsal-ventral axis) (Williams and Shepherd,
1998). This neuron may produce neuropeptides because it expresses
two genes related to neuroendocrine secretion: PHM
(peptidylglycine-alpha-hydroxylating mono-oxygenase) and dimmed (as well as the c929 PlacZ-element inserted near
the dimmed gene) (Hewes et al., 2003). |
|
development |
not described.
This neuron does not accumulate Cut
at any stage. However, achaete-scute
is required for its
formation (Dambly-Chaudiere
and Ghysen, 1987; Jarman et al., 1993). No
BrdU accumulation has been detected in this
neuron (Bodmer
et al., 1989), suggesting that
it is formed directly from a primary precursor cell without cell
division, or that its precursor cells are too deep to incorporate
BrdU. |
|
|
|
dbd = dbp |
|
sensory
organ type |
dorsal bipolar md neuron |
|
morphology
|
It emits two long dendritic branches along muscle 3 (along
the anterior-posterior axis) (Williams
and Shepherd, 1998). Its dendrites seem to join each other between
segments (Bodmer and Jan, 1989). A glial cell is
always found next to this neuron (Fredieu
and Mahowald, 1989). A lacZ
transgene under the control of derailed
5' region is expressed in only one PNS cell, the dbd-associated glial
cell (Bonkowsky and Thomas, 1999). |
|
development |
The proneural gene amos is
required for
its formation (Huang et al., 2000). The dorsoventral locations of the dbd and dmd1 neurons appear reversed relative to their amos-positive proneural clusters, suggesting that one or both neurons undergo migration during development (Holohan et al., 2006). The dbd neuron and its associated glial cell derive from the
single cell division of a primary precursor cell (Umesono
et al., 2002). This cell division requires numb, Notch and gcm (Brewster
and Bodmer, 1995; Jones et al., 1995; Umesono et al., 2002). Notch receptor is activated
in the glial cell. The
dbd axonal projection undergoes a drastic reorganization late in
embryogenesis (Schrader and Merritt, 2000; Zlatic et al.,, 2003). |
|
|
|
dp1
= p8 = dc1 (desC neuron) |
|
sensory
organ type |
dorsal mono-innervated papilla (es organ) (four cells:
socket cell, shaft cell, sheath cell, neuron) |
|
morphology
|
papilla (Dambly-Chaudiere
and Ghysen, 1986) |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby ddaF neuron. |
|
|
|
dp2
= p9 = dc2 (desD neuron) |
|
sensory
organ type |
dorsal mono-innervated papilla (es organ) (four cells:
socket cell, shaft cell, sheath cell, neuron) |
|
morphology
|
papilla (Dambly-Chaudiere
and Ghysen, 1986) |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby ddaD neuron. |
|
|
|
dh1 = h3 (desA or desB neuron) |
|
sensory
organ type |
dorsal mono-innervated hair (es organ) (four cells:
socket cell, shaft cell, sheath cell, neuron). Note however that this
organ (identified as the anterior organ in the dh1-dh2
organ pair) is reported to be tri-innervated in Campos-Ortega
and Hartenstein, 1997. |
|
morphology
|
small hair pointing towards the dorsal region (Dambly-Chaudiere
and Ghysen, 1986). Its hair is very close to the long hair of the dh2 organ. The group
of both hairs has
been named shF. |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). It may be associated by lineage to the nearby ddaA neuron. |
|
|
|
dh2 = h4 (desA = des2 neurons) |
|
sensory
organ type |
dorsal bi-innervated hair (es organ) (five cells: socket
cell, shaft cell, sheath cell, two neurons). Note however that this
organ is reported to be mono-innervated in Campos-Ortega
and Hartenstein, 1997. Also, Dambly-Chaudiere and Ghysen first
reported that this sensory organ was mono-innervated (Ghysen
et al., 1986), before indicating it was bi-innervated in their next
paper (Dambly-Chaudiere
and Ghysen, 1987). |
|
morphology
|
long hair (Dambly-Chaudiere
and Ghysen, 1986). Its hair is very close to the small hair of the dh1
organ. The group of both hairs has been named shF. |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987).
In pox-neuro mutants, dh1 is
transformed into a mono-innervated es organ located in the lateral
region (Awasaki
and Kimura, 2001). |
|
|
|
ddaF |
|
sensory
organ type |
dorsal md neuron |
|
morphology
|
Its
dendritic arborization harbours long primary and secondary
branches. Its dendrites have spiked protusions (1-20um long) along
almost their length (Sweeney et al., 2002, Grueber
et al., 2002). It
thus belongs to class III md neurons together with vdaD,
v'dap, ldaB and ddaA (Grueber
et al., 2002). Like them, it accumulates higher amounts of Cut than
other md neurons (Grueber et al., 2003). |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). Several indirect pieces of evidence suggest that it
originates from an md-es lineage. First, in hamlet
mutant, the ddaF neuron is duplicated (W.
Grueber, personal observations, see vdaB). Second, two-cell clones generated by mosaic analysis with a repressible cell marker (MARCM) often contained an es neuron and the ddaF neuron (Sweeney et al. 2002). Third, in miR-9a mutants, the ectopic ddaF neuron is associated with an ectopic Cut-positive es organ (Li et al., 2006).
Its es organ associated by lineage may be the nearby dp1
organ. |
|
|
|
ddaE |
|
sensory
organ type |
dorsal md neuron |
|
morphology
|
presents
a long single dendrite from which originate posterior directed
secondary dendrites (Sweeney et al., 2002, Grueber
et al., 2002). Its simple branching pattern places it in class I
md neurons together with vpda
and ddaD (Grueber
et al., 2002). It accumulates the class I md marker Abrupt (Sugimura
et al., 2004; Li et al., 2004). |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). Two indirect pieces of evidence suggest that it
originates from an md-es lineage. First, two-cell clones generated by mosaic analysis with a repressible cell marker (MARCM) often contained an es neuron and the ddaE neuron (Sweeney et al. 2002). Third, in miR-9a mutants, the ectopic ddaE neuron is associated with an ectopic Cut-positive es organ (Li et al., 2006). However, in hamlet
mutant, the ddaE neuron is not duplicated (W.
Grueber, personal communication, see vdaB). |
|
|
|
ddaC |
|
sensory
organ type |
dorsal md neuron |
|
morphology
|
It presents a highly complex dendritic arborization (Sweeney et al., 2002, Grueber
et al., 2002) and thus
belongs to
class
IV md neurons together with v'ada and vdaB (Grueber
et al., 2002). These three md neurons specifically accumulate the
following markers: Pickpocket
(Adams et al., 1998), Collier (Orgogozo
et al., 2004) and B6-2-25
(V. Orgogozo,
personal observations). |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). In hamlet
mutant MARCM clones, the ddaC neuron is not duplicated (W.
Grueber et al., 2003b), suggesting that it comes from an md-solo lineage (see vdaB). |
|
|
|
dda1 = dmd1 |
|
sensory
organ type |
dorsal md neuron |
|
morphology
|
Its dendritic arborization projects relatively deep below the
cuticle to trachea or muscles. |
|
development |
The proneural gene amos is
required for
its formation (Huang
et al., 2000; Brewster et al., 2001). The dorsoventral locations of the dbd and dmd1 neurons appear reversed relative to their amos-positive proneural clusters, suggesting that one or both neurons undergo migration during development (Holohan et al., 2006). The dda1/dmd1 neuron
derives from the single cell division of a primary precursor cell (Umesono
et al., 2002). Nubbin
accumulates
in this primary precursor cell as well as its progeny cells (Umesono
et al., 2002). The fate of the dmd1 sibling cell is unknown. |
|
|
|
ddaA |
|
sensory
organ type |
dorsal md neuron |
|
morphology
|
Its
dendritic arborization harbors long primary and secondary
branches. Its dendrites have spiked protrusions (1-20um long) along
almost their length (Sweeney et al., 2002, Grueber
et al., 2002). It
thus belongs to class III md neurons together with vdaD,
v'dap, ldaB and ddaF (Grueber
et al., 2002). Like them, it accumulates higher amounts of Cut than
other md neurons (Grueber et al., 2003). |
|
development |
not described. achaete-scute
is
required for its formation (Dambly-Chaudiere
and Ghysen, 1987). In hamlet
mutant, the ddaA neuron is duplicated (W.
Grueber, personal observations). This suggests that it
originates from an md-es lineage (see vdaB).
Its es organ associated by lineage may be the nearby dh1
organ. |
References
Adams, C.M., Anderson, M.G., Motto, D.G.,
Price, M.P., Johnson, W.A., Welsh, M.J., 1998. Ripped pocket and
pickpocket, novel Drosophila DEG/ENaC subunits expressed in early
development and in mechanosensory neurons. J Cell Biol. 140, pp.143-52. Medline
Awasaki,
T. and Kimura, K., 2001. Multiple function of poxn gene in larval PNS
development and in adult appendage formation of Drosophila. Dev.
Genes Evol. 211, pp. 20-29. Medline
Barolo, S., Walker, R., Polyanovsky, A.,
Freschi, G., Keil, T. and Posakony, J. W.,
2000. A Notch-independent activity of Suppressor of Hairless is
required for normal mechanoreceptor physiology. Cell 103, pp. 957-969. Medline
Bodmer,
R. and Jan, Y.N., 1987. Morphological differentiation of the embryonic
peripheral neurons in Drosophila. Roux's Arch. Dev. Biol.
196, pp. 69-77.
Bodmer,
R., Carretto, R. and Jan, Y.N., 1989. Neurogenesis of the peripheral
nervous system in Drosophila embryos: DNA replication patterns
and cell lineages. Neuron 3, pp. 21-32. Medline
Bonkowsky, J.L., Thomas, J.B., 1999. Cell-type
specific modular regulation of derailed in the Drosophila nervous
system. Mech Dev. 82, pp. 181-4. Medline
Brewster,
R. and Bodmer, R., 1995. Origin and specification of type II sensory
neurons in Drosophila. Development 121,
pp. 2923-2936. Medline
Brewster, R., Hardiman, K., Deo, M., Khan, S.
and Bodmer, R., 2001. The selector gene cut represses
a neural cell fate that is specified independently of the
Achaete-Scute-Complex and atonal. Mech
Dev. 105, pp. 57-68. Medline
Campos-Ortega, J.-A. and Hartenstein, V. 1997.
The embryonic development of Drosophila
melanogaster. Berlin/Heidelberg/New-York/Tokyo.: Springer-Verlag.
Dambly-Chaudiere, C. and Ghysen, A. 1986. The
sense organs in the Drosophila
larva and their relation to embryonic
pattern of sensory neurons. Roux's
Arch. Dev. Biol. 195,
pp.
222-228.
Dambly-Chaudiere, C. and Ghysen, A. 1987.
Independent subpatterns of sense organs require independent genes of
the achaete-scute complex in Drosophila larvae. Genes & Development
1, pp. 297-306.
Dambly-Chaudiere,
C.,
Jamet, E., Burri, M., Bopp, D., Basler, K., Hafen, E., Dumont, N.,
Spielmann, P., Ghysen, A. and Noll, M., 1992. The paired box gene pox
neuro: a determinant of poly-innervated sense organs in Drosophila.
Cell 69, pp. 159-172. Medline
Elstob, P.R., Brodu, V. and Gould, A.P. 2001.
Spalt-dependent switching between two cell fates that are induced by
the Drosophila EGF receptor. Development.
128, pp. 723-32. Medline
Fredieu, J.R and Mahowald, A.P., 1989. Glial
interactions with neurons during Drosophila embryogenesis. Development 106, pp. 739-48. Medline
Gould, A. P., Elstob, P. R. and Brodu, V.
2001. Insect oenocytes: a model system for studying cell-fate
specification by Hox genes. J Anat 199, pp. 25-33. Medline
Ghysen, A., Dambly-Chaudiere, C., Aceves, E.,
Jan, L. Y. and Jan, Y. N. 1986. Sensory neurons and peripheral pathways
in Drosophila embryos. Roux's Arch. Dev. Biol. 195, pp. 281-289.
Grueber,
W.B., Jan, L.Y. and Jan, Y.N., 2002. Tiling of the Drosophila
epidermis by multidendritic sensory neurons. Development 129,
pp. 2867-2878. Medline
Grueber, W. B., Jan, L. Y. and Jan, Y. N.
2003. Different levels of the homeodomain protein cut regulate distinct
dendrite branching patterns of Drosophila multidendritic neurons. Cell
112, pp. 805-18. Medline
Grueber, W. B., Ye B, Moore AW, Jan LY, Jan
YN. 2003. Dendrites of distinct classes of Drosophila sensory neurons
show different capacities for homotypic repulsion. Curr Biol. 2003 Apr
15;13(8):618-26. Medline
Hewes, R.S., Park, D., Gauthier, S.A.,
Schaefer, A.M., Taghert,
P.H. 2003. The bHLH protein Dimmed controls neuroendocrine cell
differentiation in Drosophila. Development
130, pp. 1771-81. Medline
Huang, M.-H., Hsu, C.-H. and Chien, C.-T.
2000. The proneural gene amos promotes multiple dendritic neuron
formation in the Drosophila peripheral nervous system. Neuron 25, pp.
57-67. Medline
Inbal, A., Levanon, D., Salzberg, A. 2003.
Multiple roles for u-turn/ventral veinless in the development of
Drosophila PNS. Development 130, pp. 2467-78. Medline
Inbal, A., Volk, T. and Salzberg, A. 2004.
Recruitment of ectodermal
attachment cells via an EGFR-dependent mechanism during the
organogenesis of Drosophila proprioceptors. Dev Cell. 7, pp.241-50. Medline
Jan,
Y.N. and Jan,
L.Y., 1993. The peripheral nervous system. In: Bate, M. and
Martinez-Arias, A., Editors, 1993. The Development of Drosophila
melanogaster vol. I, Cold Spring Harbor
Laboratory Press, New York, pp. 1207-1244.
Jarman, A. P., Grau, Y., Jan, L. Y. and Jan,
Y. N. 1993. atonal is a
proneural gene that directs chordotonal organ
formation in the Drosophila peripheral nervous system. Cell 73, pp.
1307-1321. Medline
Jones,
B.W., Fetter, R.D., Tear, G. and Goodman, C.S., 1995. Glial cells
missing: a genetic switch that controls glial versus neuronal fate. Cell
82, pp. 1013-1023. Medline
Holohan, E.E., zur Lage P.I. and Jarman A.P.,
2006. Multiple enhancers contribute to spatial but not temporal
complexity in the expression of the proneural gene, amos. BMC Dev Biol. 6, pp. 53. Medline
Lai, E.C. and Orgogozo, V., 2004. A hidden
program in Drosophila peripheral neurogenesis revealed: fundamental
principles underlying sensory organ diversity. Dev Biol. 269, pp. 1-17. Medline
Li W, Wang F, Menut L, Gao FB. 2004.
BTB/POZ-Zinc Finger Protein Abrupt Suppresses Dendritic Branching in a
Neuronal Subtype-Specific and Dosage-Dependent Manner. Neuron 43, pp.
823-34. Medline
Li Y, Wang F, Lee JA, Gao FB. 2006. MicroRNA-9a
ensures the precise specification of sensory organ precursors in
Drosophila. Genes Dev. 20, pp. 2793-805. Medline
Merritt, D. J. and Whitington, P. M. 1995.
Central projections of sensory neurons in the Drosophila embryo
correlate with sensory modality, soma position, and proneural gene
function. J Neurosci 15, pp. 1755-67. Medline
Moore AW, Jan LY, Jan YN. 2002. hamlet, a
binary genetic switch between single- and multiple- dendrite neuron
morphology. Science 297, pp.
1355-8. Medline
Okabe,
M. and Okano, H., 1997. Two-step induction of chordotonal organ
precursors in Drosophila embryogenesis. Development 124,
pp. 1045-1053. Medline
Okano,
H., Hayashi,
S., Tanimura, T., Sawamoto, K., Yoshikawa, S., Watanabe, J., Iwasaki,
M., Hirose, S., Mikoshiba, K. and Montell, C., 1992. Regulation of Drosophila
neural development by a putative secreted protein. Differentiation
52, pp. 1-11.
Orgogozo,
V.,
Schweisguth, F. and Bellaïche, Y., 2001. Lineage, cell polarity
and
inscuteable function in the peripheral nervous system of the Drosophila
embryo. Development 128, pp. 631-643. Medline
Orgogozo,
V., Schweisguth, F. and Bellaiche, Y., 2002. Binary cell death decision
regulated by unequal partitioning of Numb at mitosis. Development
129, pp. 4677-4684. Medline
Orgogozo, V., Schweisguth, F. and Bellaiche,
Y. 2004. Slit-Robo signalling prevents sensory cells from crossing the
midline in Drosophila. Mech Dev. 121, pp. 427-36. Medline
Rusten,
T.E., Cantera,
R., Urban, J., Technau, G., Kafatos, F.C. and Barrio, R., 2001. Spalt
modifies EGFR-mediated induction of chordotonal precursors in the
embryonic PNS of Drosophila promoting the development of
oenocytes. Development 128, pp. 711-722. Medline
Rutledge, B.J., Zhang, K., Bier, E., Jan, Y.N.
and Perrimon N., 1992. The Drosophila
spitz gene encodes a putative EGF-like growth factor involved in
dorsalventral axis formation and neurogenesis. Genes Dev 6, pp. 1503-1517. Medline
Schrader S. and Merritt, D.J. 2000. Central projections of Drosophila
sensory neurons in the transition from embryo to larva. J Comp Neurol. 425, pp. 34-44. Medline
Sugimura, K., Satoh, D., Estes, P., Crews, S.
and Uemura, T. 2004. Development of Morphological Diversity of
Dendrites in Drosophila by the BTB-Zinc Finger Protein Abrupt. Neuron 43, pp. 809-22. Medline
Sweeney NT, Li W, Gao FB. 2002. Genetic
manipulation of single neurons in vivo reveals specific roles of
flamingo in neuronal morphogenesis. Dev Biol 247, pp. 76-88. Medline
Umesono,
Y., Hiromi, Y. and Hotta, Y., 2002. Context-dependent utilization of
Notch activity in Drosophila glial determination. Development
129, pp. 2391-2399. Medline
Williams, D.W., Shepherd, D., 1998. Persistent
larval sensory neurons in adult Drosophila melanogaster. J Neurobiol. 39, pp. 275-86. Medline
Zlatic M, Landgraf M, Bate M. 2003. Genetic specification of axonal
arbors: atonal regulates robo3 to position terminal branches in the
Drosophila nervous system. Neuron
37, pp. 41-51. Medline
zur
Lage, P., Jan, Y.N. and Jarman, A.P., 1997. Requirement for EGF
receptor signalling in neural recruitment during formation of Drosophila
chordotonal sense organ clusters [see comments] . Curr. Biol. 7,
pp. 166-175. Medline
Comments and additions are welcome at:
Virginie.Orgogozo snv.jussieu.fr
[Valid HTML 4.01!]