 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| A1-2-29 |
|
apterous-lacZrK568 |
|
|
|||
|
|
||||||
| The
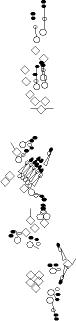
A1-2-29 enhancer-trap marker is expressed in the socket and shaft cells
of external sensory organs and in the cap and attachment cells of
chordotonal organs (Blochlinger et al., 1991; Hartenstein and Jan, 1992). This P-element is inserted upstream of the Rapgap1 gene (Kanagawa and Moore, personal communication to Flybase). |
|
After
sensory organ formation, B-galactosidase is detected at high levels in
one or two of the highly Cut-positive cells (socket or shaft cells) of
the dh1, dh2 and lh2 organ. It is also detected but at a lower level in the highly Cut-positive cells of lh1 and lp2 organs. It is not detected in the other es organs (V. Orgogozo and A.W. Moore, personal observations). |
|
|
|||
|
|
|
|
|||||
| ato-RE (not referenced in Flybase yet) | B6-2-25 | ||||||
|
|
||||||
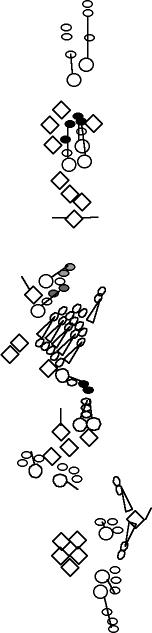
| This line contains a 367 bp fragment of the ato enhancer driving GFP. The GFP accumulates strongly in the two posterior scolopidia of the lch5 chordotonal organ and weakly in the vchB chordotonal organ (zur Lage et al., 2004). |
Same pattern as Collier and Pickpocket (V. Orgogozo and F. Schweisguth, personal observations). The md neurons that accumulate these markers are the class IV md neurons (Grueber et al., 2002). The insertion site of the P-element is unknown. | ||||||
|
|
|
|
|
|
|||
| E7-2-36 |
|
E7-3-49 |
|
gcm-lacZ P{PZ}gcmrA87 | |||
|
|
|
|||||
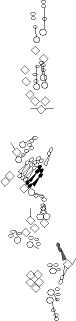
| E7-2-36 is expressed in all multidendritic neurons (Brewster and Bodmer, 1995), as well as in a single neuron of the vp5 bi-innervated organ (expression in the other bi-innervated organ dh2 has not been investigated, V. Orgogozo and F. Schweisguth, personnal observations). This P-element is inserted between the multiple and yumo genes (M.-L. Huang, S.-D. Yeh, C.-T. Chien, abstract 1049B - FlyMeeting 2003). Both encode PluA-family pseudouridine synthases. Multiple accumulates specifically in all multidendritic neurons and yumo is expressed ubiquitously during embryonic development. |
|
E7-3-49 is expressed in a subset of multidendritic neurons, as well as in the glial cell associated to dbd (Brewster and Bodmer, 1995; Brewster et al., 2001). Insertion site of the P-element is unknown. |
|
Expression is detected in ligament cells of chordotonal organs and in glial cells originating from the CNS and PNS (Jones et al., 1995). One of the glial cells is associated with the dbd neuron (Fredieu and Mahowald, 1989). Same pattern as Repo and Wrapper. | |||
|
|
|
|
|
|
|||
| scalloped-lacZETX4 |
seven up-lacZ07842 |
ppk-EGFP (not referenced in Flybase yet) | |||||
|
|
|
|||||
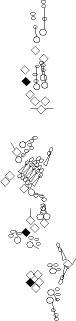
| This reporter is expressed in a subset of the PNS cells (Campbell et al., 1992). At stage 16, it is highly expressed in two PNS cells: the dbd and the vpda neurons. It is also expressed in other PNS neurons at a lower level, including dda1, ddaD, ddaE, ldaA, vbd, vdaA and the lch1, vch1 and vch2 neurons (V. Orgogozo and A.W. Moore, personal observations). |
This enhancer trap is expressed in only two PNS cell per hemisegment, the third and fifth neuron of the lch5 organ (V. Orgogozo and A.W. Moore, personal observations). | described in (Grueber et al., 2003). This marker stains the class IV md neurons. Same pattern as ppk, B6-2-25 and Collier. Low amounts of EGFP are also observed in class III neurons. EGFP expression starts in class IV neurons in stage 16 embryos and is maintained throughout larval stages and pupal stages. |
Blochlinger, K., Jan, L.Y. and Jan, Y.N., 1991. Transformation of sensory organ identity by ectopic expression of Cut in Drosophila. Genes Dev. 5, pp. 1124-1135. Medline
Brewster, R. and Bodmer, R., 1995. Origin and specification of type II sensory neurons in Drosophila. Development 121, pp. 2923-2936. Medline
Brewster, R., Hardiman, K., Deo, M., Khan, S. and Bodmer, R., 2001. The selector gene cut represses
a neural cell fate that is specified independently of the
Achaete-Scute-Complex and atonal. Mech Dev. 105, pp. 57-68. Medline
Campbell S, Inamdar M, Rodrigues V, Raghavan V, Palazzolo M, Chovnick A. 1992. The scalloped gene encodes a novel, evolutionarily conserved transcription factor required for sensory organ differentiation in Drosophila. Genes Dev. 6(3):367-79. Medline
Fredieu, J.R and Mahowald, A.P., 1989. Glial interactions with neurons during Drosophila embryogenesis. Development 106, pp. 739-48. Medline
Grueber, W.B., Jan, L.Y. and Jan, Y.N., 2002. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 129, pp. 2867-2878. Medline
Grueber, W. B., Ye B, Moore AW, Jan LY, Jan
YN. 2003. Dendrites of distinct classes of Drosophila sensory neurons
show different capacities for homotypic repulsion. Curr Biol. 2003 Apr
15;13(8):618-26. Medline
Hartenstein, V. and Jan, Y.N., 1992. Studying Drosophila embryogenesis with P-lacZ enhancer trap lines. Roux's Arch. Dev. Biol. 201, pp. 194-220
Jones, B.W., Fetter, R.D., Tear, G. and Goodman, C.S., 1995. glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell 82, pp. 1013-23. Medline
zur Lage, P.I., Powell, L.M., Prentice, D.R.A., McLaughlin, P.M. and Jarman, A.P., 2004. EGF receptor signaling triggers recruitment of Drosophila sense organ precursors by stimulating proneural gene autoregulation. Dev Cell 7, pp. 687-696. Medline